

Cystic Fibrosis in India: Past, Present and Future
* corresponding author(s):.
- Lorem ipsum dolor sit amet, adipiscing elitazaa.
- Lorem ipsum dolor sit amet, consectetur adipiscing elitazaa.
- Lorem ipsum dolor sit amet, consectetur elitazaa.
- Lorem ipsum dolor sit, adipiscing elitazaa.
INTRODUCTION
Magnitude of problem, estimated burden of cf in india, genetics and molecular defect, clinical manifestations.
Nasal Transepithelial Potential Difference (NPD) is a labor intensive and technically difficult method available only at small numbers of CF research centers. It remains to be a research tool and has limited validation as a diagnostic test [57].
Future therapies
Prognosis of cf, research priorities.
- More number of mutation studies in Indian CF patients in an attempt to find out the full spectrum of CF mutation in this country, so that a comprehensive diagnostic panel could be formulated.
- To develop simpler ancillary tests, e.g., aquagenic palmer wrinkling [75] to aid the diagnosis.
- To formulate guidelines for daily salt supplementation in hot and humid climate like India.
- To discover newer and cheaper antibiotics, that can be given through inhaled route achieving higher lung concentrations, especially against resistant pathogens like Pseudomonas .
SUMMARY AND CONCLUSION
- Cystic Fibrosis Trust (2015) History of Cystic Fibrosis. Cystic Fibrosis Trust, London, UK.
- Andersen DH (1938) Cystic fibrosis of the pancreas and its relation to celiac disease: A clinical and pathologic study. Am J Dis Child. 56: 344-399.
- Di Sant’agnese P, Darling RC, Perara GA, Shea E (1953) Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas. AMA Am J Dis Child 86: 618-619.
- Gibson LE, Cooke RE (1959) A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 23: 545-549.
- Amaral MD, Balch WE (2015) Hallmarks of therapeutic management of the cystic fibrosis functional landscape. J Cyst Fibros 14: 687-699.
- Ong TJ, Mehta A, Ogston S, Mukhopadhyay S (1998) Prediction of lung function in the inadequately nourished. Arch Dis Child. 79: 18-21.
- Cystic Fibrosis Foundation (2008) Patient Registry, Annual Data Report 2007. Cystic Fibrosis Foundation, Bethesda, USA.
- Dodge JA, Lewis PA, Stanton M, Wilsher J (2007) Cystic fibrosis mortality and survival in the UK: 1947-2003. Eur Respir J 29: 522-526.
- Dodge JA, Morison S, Lewis PA, Coles EC, Geddes D, et al. (1997) Incidence, population, and survival of cystic fibrosis in the UK, 1968-95. UK Cystic Fibrosis Survey Management Committee. Arch Dis Child 77: 493-496.
- Hamosh A, FitzSimmons SC, Macek M, Knowles MR, Rosenstein BJ, et al. (1998) Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J Pediatrics 132: 255-259.
- FitzSimmons S (1993) The changing epidemiology of cystic fibrosis. J Pediatr 122: 1-9.
- Bhakoo ON, Kumar R, Walia BNS (1968) Mucoviscidosis of lungs. Report of a case. Indian J Pediatr 35: 183-185.
- Mehta S, Wadhwa UN, Mehta SK, Chhuttani PN (1968) Fibrocystic disease of pancreas in India. Indian Pediatr 5: 185-191.
- Mehta S, Ansari Z, Wadhwa UN, Walia BN (1969) Fibrocystic disease of pancreas. Indian Pediatr 6: 114-117.
- Devi CS, Rao NR, Ramaiah Y (1969) Cystic fibrosis of pancreas in adults. A report of 4 cases. Indian J Chest Dis 11: 163-167.
- Gupte SP, Mehta S (1970) Chronic diarrhoea--an etiological study. Indian Pediatr 7: 625-627.
- Reddy CR, Sita Devi C, Anees AM, Prasantha Murthy D, Eswar Reddy G (1970) Cystic fibrosis of pancreas in India. J Trop Med Hyg 73: 59-62.
- Venkataramana G, Kousalya L, Reddy CR (1972) Cystic fibrosis of the pancreas. Indian J Pediatr 39: 337-338.
- Maya PP, Hill PG, Sudarsanam D, Jadhav M (1980) Cystic fibrosis in South India. Trop Geogr Med 32: 45-49.
- Jagadish JS (1989) Cystic fibrosis of the lungs. Indian J Pediatr 56: 288-290.
- Prasad ML, Misra A, Mathur M, Gupta DK (1990) Cystic fibrosis: postmortem report on two cases. Indian Pediatr 27: 493-496.
- Deivanayagam CN, Venogupalan K, Mallikensan S, Madhavan K, Muthukumaraswamy N (1990) A Clinical profile of cystic fibrosis in South India. Lung India 8: 167-72.
- Sarkar AK, Bag SK, Biswas SK, Saha SG (1992) Acro-osteolysis of phalanges in a case of cystic fibrosis. Indian J Pediatr 59: 636-639.
- Kabra SK, Kabra M, Ghosh M, Verma IC (1996) Cystic fibrosis--an Indian perspective on recent advances in diagnosis and management. Indian J Pediatr 63: 189-198.
- Kabra M, Ghosh M, Kabra SK, Khanna A, Verma IC (1996) Delta F508 molecular mutation in Indian children with cystic fibrosis. Indian J Med Res 104: 355-358.
- Kabra SK, Kabra M, Ghosh M, Khanna A, Pandey RM (1999) Cystic fibrosis in Indian children: clinical profile of 62 children. Pediatr Pulmonol 19: 337.
- Kabra M, Kabra SK, Ghosh M, Khanna A, Arora S, et al. (2000) Is the spectrum of mutations in Indian patients with cystic fibrosis different? Am J Med Genet 93: 161-163.
- Singh M, Prasad R, Kumar L (2002) Cystic fibrosis in North Indian children. Indian J Pediatr 69: 627-629.
- Kabra SK, Kabra M, Lodha R, Shastri S, Ghosh M, et al. (2003) Clinical profile and frequency of delta f508 mutation in Indian children with cystic fibrosis. Indian Pediatr 40: 612-619.
- Ashavaid TF, Dherai AJ, Kondkar AA, Raghavan R, Udani SV, et al. (2003) Molecular diagnosis of cystic fibrosis in Indian patients--a preliminary report. J Assoc Physicians India 51: 345-348.
- Ashavaid TF, Kondkar AA, Dherai AJ, Raghavan R, Udani SV, et al. (2005) Application of multiplex ARMS and SSCP/HD analysis in molecular diagnosis of cystic fibrosis in Indian patients. Mol Diagn 9: 59-66.
- Sharma N, Singh M, Kaur G, Thapa BR, Prasad R (2009) Identification and characterization of CFTR gene mutations in Indian CF patients. Ann Hum Genet 73: 26-33.
- Mir TA, Ashraf M, Ahmed K, Chowdhary J, Rehana B, et al. (2011) Clinical profile, diagnostic delay, and genetic make-up of cystic fibrosis in Kashmir, India. Lung India 28: 97-100.
- Santra G, Banerjee S (2012) Adult cystic fibrosis--a rare diagnosis from India. J Assoc Physicians India 60: 45-47.
- Kawoosa MS, Bhat MA, Ali SW, Hafeez I, Shastri S (2014) Clinical and mutation profile of children with cystic fibrosis in Jammu and Kashmir. Indian Pediatr 51: 185-189.
- Chakraborty PP, Ray S, Bhattacharjee R, Ghosh S, Mukhopadhyay P, et al. (2015) Diabetes and primary infertility in young males: do not forget cystic fibrosis. Clin Diabetes 33: 80-83.
- Sharma P, Arthi N, Bhattad S, Vaiphei K (2015) Cystic fibrosis in a retro-positive child. Indian J Pathol Microbiol 58: 204-210.
- Spencer DA, Venkataraman M, Higgins S, Stevenson K, Weller PH (1994) Cystic fibrosis in children from ethnic minorities in the West Midlands. Respir Med 88: 671-675.
- Powers CA, Potter EM, Wessel HU, Lloyd-Still JD (1996) Cystic fibrosis in Asian Indians. Arch Pediatr Adolesc Med 150: 554-555.
- Kapoor V, Shastri SS, Kabra M, Kabra SK, Ramachandran V, et al. (2006) Carrier frequency of F508del mutation of cystic fibrosis in Indian population. J Cyst Fibros 5: 43-46.
- Shah U, Frossard P, Moatter T (2009) Cystic fibrosis: defining a disease under-diagnosed in Pakistan. Trop Med Int Health 14: 542-545.
- Wikipedia contributors (2015) Demographics of India. Wikipedia, The Free Encyclopedia, San Fransico, USA.
- Frizzell RA (1995) Functions of the cystic fibrosis transmembrane conductance regulator protein. Am J Respir Crit Care Med 151: 54-58.
- CFMDB Statistics. Cystic Fibrosis Mutation database.
- Wilmott RW (1998) Making the diagnosis of cystic fibrosis. J Pediatr 132: 563-565.
- Ashavaid TF, Raghavan R, Dhairyawan P, Bhawalkar S (2012) Cystic fibrosis in India: a systematic review. J Assoc Physicians India 60: 39-41.
- Kabra SK, Kabra M, Lodha R, Shastri S (2007) Cystic fibrosis in India. Pediatr Pulmonol 42: 1087-1094.
- Shastri SS, Kabra M, Kabra SK, Pandey RM, Menon PS (2008) Characterisation of mutations and genotype-phenotype correlation in cystic fibrosis: experience from India. J Cyst Fibros 7: 110-115.
- Sachdeva K, Saxena R, Puri R, Bijarnia S, Kohli S, et al. (2012) Mutation analysis of the CFTR gene in 225 children: identification of five novel severe and seven reported severe mutations. Genet Test Mol Biomarkers 16: 798-801.
- Yadav K, Singh M, Angurana SK, Attri SV, Sharma G, et al. (2014) Evaluation of micronutrient profile of North Indian children with cystic fibrosis: a case-control study. Pediatr Res 75: 762-766.
- Agarwal G, Kapil A, Kabra SK, Das BK, Dwivedi S (2005) Characterization of Pseudomonas aeruginosa isolated from chronically infected children with cystic fibrosis in India. BMC Microbiol 21: 5-43.
- Chakrabarty B, Kabra SK, Gulati S, Toteja GS, Lodha R, et al. (2013) Peripheral neuropathy in cystic fibrosis: a prevalence study. J Cyst Fibros 12: 754-760.
- Sharma VK, Raj D, Xess I, Lodha R, Kabra SK (2014) Prevalence and risk factors for allergic bronchopulmonary aspergillosis in Indian children with cystic fibrosis. Indian Pediatr 51: 295-297.
- Moran A, Dunitz J, Nathan B, Saeed A, Holme B, et al. (2009) Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 32: 1626-1631.
- Midha S, Khajuria R, Shastri S, Kabra M, Garg PK (2010) Idiopathic chronic pancreatitis in India: phenotypic characterisation and strong genetic susceptibility due to SPINK1 and CFTR gene mutations. Gut 59: 800-807.
- Sharma H, Mavuduru RS, Singh SK, Prasad R (2014) Increased frequency of CFTR gene mutations identified in Indian infertile men with non-CBAVD obstructive azoospermia and spermatogenic failure. Gene 548: 43-47.
- Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, et al. (2008) Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J pediatr 153: 2-14.
- Lebecque P, Leal T, De Boeck C, Jaspers M, Cuppens H, et al. (2002) Mutations of the cystic fibrosis gene and intermediate sweat chloride levels in children. Am J Respir Crit Care Med 165: 757-761.
- Rosenstein BJ, Cutting GR (1998) The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr 132: 589-595.
- LeGrys VA (2001) Assessment of sweat-testing practices for the diagnosis of cystic fibrosis. Arch Pathol Lab Med 125: 1420-1424.
- LeGrys VA (1996) Sweat testing for the diagnosis of cystic fibrosis: practical considerations. J Pediatr 129: 892-897.
- Menon P (2012) Cystic fibrosis, are we missing in India? Int J Pharm Sci Rev Res 3: 477-481.
- Kabra SK, Kabra M, Gera S, Lodha R, Sridevi KN, et al. (2002) An indigenously developed method for sweat collection and estimation of chloride for diagnosis of cystic fibrosis. Indian Pediatr 39: 1039-1043.
- Schales O, Schales SS (1941) A simple and accurate method for determination of chloride in biological fluids. J Biol Chem 140: 879-884.
- Stern RC, Boat TF, Doershuk CF (1982) Obstructive azoospermia as a diagnostic criterion for the cystic fibrosis syndrome. Lancet 1: 1401-1404.
- Littlewood JM, Wolfe SP (2000) Control of malabsorption in cystic fibrosis. Paediatr Drugs 2: 205-222.
- Daftary A, Acton J, Heubi J, Amin R (2006) Fecal elastase-1: utility in pancreatic function in cystic fibrosis. J Cyst Fibros 5: 71-76.
- Sinaasappel M, Stern M, Littlewood J, Wolfe S, Steinkamp G, et al. (2002) Nutrition in patients with cystic fibrosis: a European Consensus. J Cyst Fibros 1: 51-75.
- Wark P, McDonald VM (2009) Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev 2: 001506.
- Derichs N (2013) Targeting a genetic defect: cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis. Eur Respir Rev 22: 58-65.
- Quittner A, Suthoff E, Rendas-Baum R, Bayliss MS, Sermet-Gaudelus I, et al. (2015) Effect of ivacaftor treatment in patients with cystic fibrosis and the G551D-CFTR mutation: patient-reported outcomes in the STRIVE randomized, controlled trial. Health Qual Life Outcomes 13: 93-101.
- Kuk K, Taylor-Cousar JL (2015) Lumacaftor and ivacaftor in the management of patients with cystic fibrosis: current evidence and future prospects. Ther Adv Respir Dis 9: 313-326.
- Wilschanski M, Miller LL, Shoseyov D, Blau H, Rivlin J, et al. (2011) Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J 38: 59-69.
- Patrick Lebecque (2012) The Prognosis of Cystic Fibrosis - A Clinician’s Perspective. In: Sriramulu D (ed.). Cystic Fibrosis-Renewed Hopes Through Research. Intech, Rijeka, Croatia.
- Gild R, Clay CD, Morey S (2010) Aquagenic wrinkling of the palms in cystic fibrosis and the cystic fibrosis carrier state: a case-control study. Br J Dermatol 163: 1082-1084.
Citation: Mandal A, Kabra SK, Lodha R (2015) Cystic Fibrosis in India: Past, Present and Future. J Pulm Med Respir Res 1: 002.
Copyright: © 2015 Anirban Mandal, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Pulmonology Pulmonary Medicine Cystic Fibrosis Chronic Obstructive Pulmonary Disease (COPD) Lung Diseases
Advertisement
Diagnosis and treatment of cystic fibrosis in India: What is at stake for developing countries?
- Published: 15 June 2024
- Volume 49 , article number 64 , ( 2024 )
Cite this article

- Anand Kumar Purushothaman 1 ,
- Srikanth Natarajan 1 ,
- Trailokyanath Panigrahi 1 &
- Everette Jacob Remington Nelson ORCID: orcid.org/0000-0002-9781-526X 1
116 Accesses
1 Altmetric
Explore all metrics
Cystic fibrosis (CF) is a life-threatening monogenic disease affecting thousands of people worldwide. Cystic fibrosis transmembrane conductance regulator (CFTR) is an ion channel that facilitates transportation of water and salts across epithelial cell membranes through the conductance of Cl − and other anions. A dysfunctional CFTR due to abnormalities in the cftr gene causes CF, which is believed to be a rare disease in India mainly due to mis/underdiagnosis. Although numerous diagnostic methods and treatment options are available for CF globally, most of these are unaffordable for developing countries like India. Currently, CF symptoms are managed with mucolytics, antibiotics, anti-inflammatory drugs, and various CFTR modulators based on the type of defect. While a definitive cure for CF remains elusive, advancements in stem cell and gene therapies hold promise for permanent cure in the near future. In this review, we discuss the prevalence of CF cases in India, affordable diagnostic methods, and treatment options amenable for developing countries. We further emphasize the scope for the universal newborn screening programme.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Cystic Fibrosis in Adults

Cystic Fibrosis: Biology and Therapeutics
Cystic fibrosis.
Baker MW, Atkins AE, Cordovado SK, et al . 2016 Improving newborn screening for cystic fibrosis using next-generation sequencing technology: a technical feasibility study. Genet. Med. 18 231–238
Article CAS PubMed Google Scholar
Bhakoo ON, Kumar R and Walia BNS 1968 Mucoviscidosis of the lung. Indian J. Pediatr. 35 183–185
Boeck KD, Munck A, Walker S, et al . 2014 Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J. Cyst. Fibros. 13 674–680
Article PubMed Google Scholar
Bulcha JT, Wang Y, Ma H, et al . 2021 Viral vector platforms within the gene therapy landscape. Sig. Transduct. Target Ther. 6 1–24
Article Google Scholar
Carraro G, Perin L, Sedrakyan S, et al . 2008 Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells 26 2902–2911
Chamayou S, Sicali M, Lombardo D, et al . 2020 Universal strategy for preimplantation genetic testing for cystic fibrosis based on next generation sequencing. J. Assist. Reprod. Genet. 37 213–222
Clancy JP, Rowe SM, Accurso FJ, et al . 2012 Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 67 12–18
Comeau AM, Accurso FJ, White TB, et al . 2007 Guidelines for implementation of cystic fibrosis newborn screening programs: Cystic Fibrosis Foundation Workshop Report. Pediatrics 119 495–518
Condren ME and Bradshaw MD 2013 Ivacaftor: A novel gene-based therapeutic approach for cystic fibrosis. J. Pediatr. Pharmacol. Ther. 18 8–13
PubMed PubMed Central Google Scholar
Cystic Fibrosis Foundation 2021 Annual Data Report ( https://www.cff.org/sites/default/files/2021-11/Patient-Registry-Annual-Data-Report.pdf )
da Silva Filho LVRF, Zampoli M, Cohen-Cymberknoh M, et al . 2021 Cystic fibrosis in low and middle-income countries (LMIC): A view from four different regions of the world. Paediatr. Respir. Rev. 38 37–44
PubMed Google Scholar
Du M, Jones JR, Lanier J, et al . 2002 Aminoglycoside suppression of a premature stop mutation in a Cftr –/– mouse carrying a human CFTR-G542X transgene. J. Mol. Med. 80 595–604
Du M, Keeling KM, Fan L, et al . 2006 Clinical doses of amikacin provide more effective suppression of the human CFTR-G542X stop mutation than gentamicin in a transgenic CF mouse model. J. Mol. Med. 84 573–582
Farrell PM, Kosorok MR, Laxova A, et al . 1997 Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. N. Engl. J. Med. 337 963–969
Farrell PM, Kosorok MR, Rock MJ, et al . 2001 Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Pediatrics 107 1–13
Farrow N, Miller D, Cmielewski P, et al . 2013 Airway gene transfer in a non-human primate: Lentiviral gene expression in marmoset lungs. Sci. Rep. 3 1287
Article CAS PubMed PubMed Central Google Scholar
Flotte TR, Zeitlin PL, Reynolds TC, et al . 2003 Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum. Gene Ther. 14 1079–1088
Flotte TR, Ng P, Dylla DE, et al . 2007 Viral vector–mediated and cell-based therapies for treatment of cystic fibrosis. Mol. Ther. 15 229–241
Flume PA, Liou TG, Borowitz DS, et al . 2012 Ivacaftor in subjects with cystic fibrosis who are homozygous for the f508del-CFTR mutation. CHEST 142 718–724
Article PubMed PubMed Central Google Scholar
Ghosh S, Brown AM, Jenkins C, et al . 2020 Viral Vector systems for gene therapy: a comprehensive literature review of progress and biosafety challenges. Appl. Biosaf. 25 7–18
Guo J, Garratt A and Hill A 2022a Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J. Cyst. Fibros. 21 456–462
Guo J, Wang J, Zhang J, et al . 2022b Current prices versus minimum costs of production for CFTR modulators. J. Cyst. Fibros. 21 866–872
Hida K, Lai SK, Suk JS, et al . 2011 Common gene therapy viral vectors do not efficiently penetrate sputum from cystic fibrosis patients. PLoS One 6 19919
Jat KR 2013 Spirometry in children. Prim. Care. Respir. J 22 221–229
Johnson C, Butler SM, Konstan MW, et al . 2003 Factors influencing outcomes in cystic fibrosis: a center-based analysis. CHEST 123 20–27
Kabra SK, Kabra M, Connett GJ, et al . 1999 Diagnosis of cystic fibrosis: Indian perspective. Indian J. Pediatr. 66 923–928
Kabra M, Kabra SK, Ghosh M, et al . 2000 Is the spectrum of mutations in Indian patients with cystic fibrosis different? Am. J. Med. Genet. 93 161–163
Kabra SK, Kabra M, Gera S, et al . 2002 An indigenously developed method for sweat collection and estimation of chloride for diagnosis of cystic fibrosis. Indian Pediatr. 39 1039–1043
CAS PubMed Google Scholar
Kabra SK, Kabra M, Lodha R, et al . 2003 Clinical profile and frequency of Delta F508 mutation in Indian children with cystic fibrosis. Indian Pediatr. 40 612–619
Kabra SK, Kabra M, Lodha R, et al . 2007 Cystic fibrosis in India. Pediatr. Pulmonol. 42 1087–1094
Kerem E, Konstan MW, Boeck KD, et al . 2014 Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir. Med. 2 539–547
Kim N, Duncan GA, Hanes J, et al . 2016 Barriers to inhaled gene therapy of obstructive lung diseases: A review. J. Control. Release. 240 465–488
Kiseleva A, Klimushina M, Sotnikova E, et al . 2020 Cystic fibrosis polymorphic variants in a Russian population. Pharmgenomics Pers. Med. 13 679–686
CAS PubMed PubMed Central Google Scholar
Konstan MW, Butler SM, Wohl MEB, et al . 2003 Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J. Pediatr. 142 624–630
Lands LC and Dauletbaev N 2010 High-dose Ibuprofen in cystic fibrosis. Pharmaceuticals 37 2213–2222
LeGrys VA, Yankaskas JR, Quittell LM, et al . 2007 Diagnostic sweat testing: the cystic fibrosis foundation guidelines. J. Pediatr. 151 85–89
Mandal A, Kabra SK and Lodha R 2015 Cystic fibrosis in India: Past, present and future. J. Pulm. Med. Respir. Res. 1 1–8
Google Scholar
Mir TA, Ashraf M, Ahmed K, et al . 2011 Clinical profile, diagnostic delay, and genetic make-up of cystic fibrosis in Kashmir, India. Lung India 28 97
Mishra A, Greaves R, Smith K, et al . 2008 Diagnosis of cystic fibrosis by sweat testing: age-specific reference intervals. J. Pediatr. 153 758–763
Mohite PN, Dave K, Reed A, et al. 2021 Lung transplantation in patients with cystic fibrosis; in Cystic fibrosis - Facts, management and advances (Eds.) P Mohite, A Reed and AR Simon (IntechOpen) ( https://www.intechopen.com/chapters/74734 )
Mookken T 2020 Universal implementation of newborn screening in India. Int. J. Neonatal Screen. 6 24
Mueller C and Flotte TR 2008 Gene therapy for cystic fibrosis. Clin. Rev. Allergy Immunol. 35 164–178
O’Sullivan BP and Freedman SD 2009 Cystic fibrosis. Lancet 373 1891–1904
Padman R, McColley SA, Miller DP, et al . 2007 Infant care patterns at epidemiologic study of cystic fibrosis sites that achieve superior childhood lung function. Pediatrics 119 531–537
Pagin A, Devos A, Figeac M, et al . 2016 Applicability and efficiency of NGS in routine diagnosis: in-depth performance analysis of a complete workflow for CFTR mutation analysis. PLoS One 11 0149426
Paracchini V, Carbone A, Colombo F, et al . 2012 Amniotic mesenchymal stem cells: a new source for hepatocyte-like cells and induction of CFTR expression by coculture with cystic fibrosis airway epithelial cells. J. Biomed. Biotechnol. 2012 575471
Petersen TH, Calle EA, Zhao L, et al . 2010 Tissue-engineered lungs for in vivo implantation. Science 329 538–541
Price AR, Limberis MP, Wilson JM, et al . 2007 Pulmonary delivery of adenovirus vector formulated with dexamethasone–spermine facilitates homologous vector re-administration. Gene Ther. 14 1594–1604
Ren HY, Grove DE, De La Rosa O, et al . 2013 VX-809 corrects folding defects in cystic fibrosis transmembrane conductance regulator protein through action on membrane-spanning domain 1. MBoC 24 3016–3024
Riordan JR, Rommens JM, Kerem BS, et al . 1989 Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245 1066–1073
Roy V, Gupta M and Ghosh RK 2015 Perception, attitude and usage of complementary and alternative medicine among doctors and patients in a tertiary care hospital in India. Indian J. Pharmacol. 47 137–142
Sahoo N, Dhochak N, Jat KR, et al . 2022 Development of algorithm for diagnosis of cystic fibrosis in absence of sweat chloride testing in resource-limited setting. Pediatr. Pulmonol. 57 3077–3083
Sarkar A 2002 Cystic fibrosis: Indian experience. Indian Pediatr. 39 813–818
Scotet V, Gutierrez H and Farrell PM 2020 Newborn screening for CF across the globe-Where is it worthwhile? Int. J. Neonatal Screen. 6 18
Shastri SS, Kabra M, Kabra SK, et al . 2008 Characterisation of mutations and genotype–phenotype correlation in cystic fibrosis: Experience from India. J. Cyst. Fibros. 7 110–115
Singh A, Lodha R, Shastri S, et al . 2019 Aquagenic wrinkling of skin: a screening test for cystic fibrosis. Indian Pediatr. 56 109–1013
Sinn PL, Cooney AL, Oakland M, et al . 2012 Lentiviral vector gene transfer to porcine airways. Mol. Ther. Nucleic Acids 1 e56
Therrell BL, Lloyd-Puryear MA and Mann MY 2005 Understanding newborn screening system issues with emphasis on cystic fibrosis screening. J. Pediatr. 147 S6–S10
Thibodeau PH, Richardson JM, Wang W, et al . 2010 The cystic fibrosis-causing mutation ΔF508 affects multiple steps in cystic fibrosis transmembrane conductance regulator biogenesis. J. Biol. Chem. 285 35825–35835
Van Goor F, Hadida S, Grootenhuis PDJ, et al . 2011 Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 108 18843–18848
Veit G, Avramescu RG, Chiang AN, et al . 2016 From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol. Biol. Cell. 27 424–433
Wang G, Bunnell BA, Painter RG, et al . 2005 Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: Potential therapy for cystic fibrosis. Proc. Natl. Acad. Sci. USA 102 186–191
Waters V and Smyth A 2015 Cystic fibrosis microbiology: Advances in antimicrobial therapy. J. Cyst. Fibros. 14 551–560
Welsh MJ, Ramsey BW, Accurso F, et al. 2019 Cystic fibrosis; in The online metabolic and molecular bases of inherited disease DL Valle (eds) Antonarakis S and Ballabio A, et al. (New York: McGraw-Hill Education)
Download references
Author information
Authors and affiliations.
Gene Therapy Laboratory, School of Bio Sciences and Technology, Vellore Institute of Technology, Vellore, India
Anand Kumar Purushothaman, Srikanth Natarajan, Trailokyanath Panigrahi & Everette Jacob Remington Nelson
You can also search for this author in PubMed Google Scholar
Contributions
AKP reviewed the literature, drafted the manuscript, and conceptualized figures; SN drafted the manuscript and completed figures; TP conceptualized and drafted the manuscript; and EJRN conceptualized and extensively revised the manuscript and figures.
Corresponding author
Correspondence to Everette Jacob Remington Nelson .
Ethics declarations
Conflict of interest.
The authors do not have any conflicts of interest to disclose.
Additional information
Corresponding editor: R akesh K M ishra
This article is part of the Topical Collection: The Rare Genetic Disease Research Landscape in India.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Purushothaman, A.K., Natarajan, S., Panigrahi, T. et al. Diagnosis and treatment of cystic fibrosis in India: What is at stake for developing countries?. J Biosci 49 , 64 (2024). https://doi.org/10.1007/s12038-024-00456-5
Download citation
Received : 07 November 2023
Accepted : 09 May 2024
Published : 15 June 2024
DOI : https://doi.org/10.1007/s12038-024-00456-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cystic fibrosis
- diagnostic methods
- newborn screening
- treatment options
- Find a journal
- Publish with us
- Track your research

CYSTIC FIBROSIS SCENARIO IN INDIA
Article sidebar, main article content.
Abnormal transport of chloride ion in the epithelial cells is caused by a autosomal recessive monogenic condition known as cystic fibrosis (CF). It belongs to the rare genetic disease in India. Persistent coughing with phlegm, pneumonia, bronchitis, bulky stool and hard bowl movement are most symptoms of the disease. Mutation in CFTR (cystic fibrosis transmembrane conductance regulator) gene present on chromosome 7 having 230 kb nucleotides with 23 exons leads to development of disease. Determination of sweat electrolyte is considered as optimal diagnostic method. Results of previous studies have shown that cystic fibrosis increase the sodium and chloride concentrations. Mutations like ΔF508, G542X, R553X, N130K and 621+1 (G →T) are most common in CF patients in India. Among them ΔF508 is most severe and predominant mutation. Reports have shown as high as 56% frequency of ΔF508 in Indian patients. CF can be treated with anti-inflammatory drugs, CFTR modulators and combination therapies. Early screening can be an effective strategy for early diagnosis and treatment of cystic fibrosis.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License .
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Molecular Basis of Cystic Fibrosis Disease: An Indian Perspective
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Issue date 2010 Oct.
Cystic fibrosis is a common autosomal recessive disorder usually found in population of white Caucasian descent. Now it is well documented the presence of CF disease in India with the advancement of laboratory testing. As once it was thought non existence of this disease in our population. Most of the phenotype of CF disease was in accordance of western population. Genetic analysis of CFTR gene in Indian CF patients revealed that most common mutation was delta F508 mutation. However, it was less than Caucasian population. CFTR mutations are also a causative factor in the pathogenesis of male infertility due to obstructive azoospermia. There are two most common mutation viz. IVS8-T5 and delta F508 which are responsible for congenital absence of vas deferens in male infertility patients. Elevated levels of sweat chloride at two occasions along with the presence of two mutations in CFTR gene was gold standard method for diagnosis of CF disease. It is noteworthy here that due to magnitude of Indian population, the total CF disease load would be more than many European countries. Clinical data demonstrate the prevalence of both classical and genetic form of CF in India.
Keywords: Cystic fibrosis, CFTR, Delta F508, Congenital absence of vas deferens (CAVD)
Introduction
In the last two decades, biochemical genetic disorders of metabolism are of great importance due to improved molecular genetic analysis and biochemical testing. Other reason for emphasis on genetic disorders is due to more admission of children at Pediatric Ward. Presently, children deaths due to infectious diseases are reduced to great extent at Indian scenario. We established the relative frequencies of various genetic disorders at PGIMER, Chandigarh. Cystic fibrosis was found with 7.56 frequency [ 1 ]. Notwithstanding, CF is a common autosomal recessive disorder usually found in population of White Caucasian descent, such as those of Europe, North America and Australia. CF like other genetic disorders has geographical variation in the epidemiology, clinical presentation and course. Before seventies, CF was considered as non existent in India. First case of CF disease was reported in 1968 from PGIMER, Chandigarh [ 2 ]. Later, suggested incidence of cystic fibrosis (CF) in Asian migrants (Indians and Pakistanis) in the United Kingdom was reported about 1 in 10,800 to 12,000 [ 3 , 4 ]. Nevertheless, there have been many publications describing diverse and unique manifestations of the disease in Indian population. The present review is intended to provide a point of reference at national organization and to help them in planning, diagnostic and genetic analysis for affected patients and their families. Since a better awareness of CF and the increasing availability diagnostic tests—the sweat chloride and/or DNA tests frequently leads to the identification of a higher number of affected individuals.
Cystic fibrosis was first recognized by Dorothy Andersen in 1938 [ 5 ] as cystic fibrosis of the pancreas and its relation to celiac disease. Farber in 1945 [ 6 ] recognized the implication of sticky mucus in manifestation of symptoms, and termed as mucoviscidosis. Acute salt loss caused by excessive sweating in babies with CF during heat waves was demonstrated by Di Sant’Agnese in 1953 [ 7 ]. Subsequently, Pilocarpine ionotophoresis method was developed for sweat collection and Chloride measurement. Altered electrical properties with abnormalities of both sodium and chloride transport was described by Knowles et al. [ 8 ]. CF gene was mapped to a small segment of long arm of chromosome 7q using restriction fragment length polymorphism. Linkage disequilibrium was observed between the markers XV2C/KM19 [ 9 ] and cystic fibrosis [ 10 ]. Later CF gene was cloned and C-DNA sequence was documented [ 11 ].
Prevalence and Clinical Manifestations of CF Disease
The estimated gene frequency of cystic fibrosis in the Caucasians is approximately 1 in 2,500. It also affects other ethnic groups such as black population with an incidence of 1 in 17,000 and the native American population with an approximate incidence of 1 in 80,000. The estimated incidence of CF in Asians (Indians and Pakistanis) migrated to UK is about 1:10,000 to 1:12,000. However a Similar data for Indian population is not available. In an extensive literature search, we could find a few case reports from India (Table 1 ). Cystic fibrosis was thought to be extremely rare in India. However, Published reports indicate that CF is probably far more common in people of Indian origin than previously thought but is under diagnosed or missed in majority of cases.
Cystic fibrosis in India
At PGIMER Chandigarh 100 cases of classical CF were diagnosed by us according to clinical criteria sweat testing and molecular diagnosis over the last 7 years out of which 50 were adult CF infertile males [ 12 ]. The incidence in migrant Indian population in USA has been estimated to be 1 in 40,000 [ 4 ] and in the UK between 1 in 10,000 to 12,000 [ 13 ]. The median age of diagnosis among Indian American is 12 months compared with 6 months among Caucasians American children. Kabra et al. suggested that genetic and clinical profile on Indian children with CF may be different [ 14 ]. Kapoor et al. reported a carrier rate as 0.4% on 955 cord samples and estimated its incidence to be 1 in 40,000 [ 15 ].
Classical CF characterized by progressive lung disease, pancreatic dysfunction, elevated sweat chloride electrolyte, meconiumileus and male infertility [ 16 ]. However a wide variability in clinical expression is found among patients. Upto 20% of affected infant are born intestinal obstruction. Other patients are diagnosed with various mode of presentation from birth to adulthood and with considerable variability in the severity and rate of disease progression. Notwithstanding progressive lung disease is the most common cause mortality in CF. There is a great variability in the age of onset and severity of lung disease in different age groups.
Our data on Clinical profile in Indian patients of classical CF shows that 94% had history of failure to thrive, 82% malabsorption, 90% chronic cough and 74% had recurrent or persistent pneumonia at the time of presentation, both respiratory and digestive symptoms were 60% more common as compared to either respiratory 30% or digestive symptoms 10% (Fig. 1 ). Congenital bilateral absence of vas deferens (CBAVD) occurs in 1–2% of infertile but otherwise healthy men [ 17 ]. CBAVD is present in more than 95% of cystic fibrosis (CF) males. Different studies have shown a high frequency of cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in CBAVD patients [ 18 – 21 ]. In intron 8 of the CFTR gene, the T5 allele, in contrast with the other two alleles, T7 and T9, leads to a high proportion of mRNA transcripts lacking exon 9. Consequently, the T5 variant produces abnormally low levels of CFTR protein. The T5 variant is the most frequent mutation associated with the CBAVD phenotype [ 22 ]. Low frequencies of CFTR mutations have been detected in patients with unilateral absence of vas deferens (CUAVD) [ 23 ]. Between 11 and 26% of patients with congenital absence of vas deferens (CAVD) have renal agenesis in association with it and initial negative results in the analysis of CFTR mutations in these patients suggested that urogenital anomalies have a different etiology to isolated CAVD [ 24 ]. Little is known about the spectrum and frequency of CFTR gene mutations in India. Our recent study as well as few other investigations have provided evidence for an extensive allelic heterogeneity in Indian patients with classic CF [ 25 – 27 ]. Congenital absence of vas deferens, as a distinct clinical entity with regard to CFTR mutations, has never been investigated in our population. We recently investigated the family history of infertility among CF subjects and characterized mutations in infertile members [ 12 ].
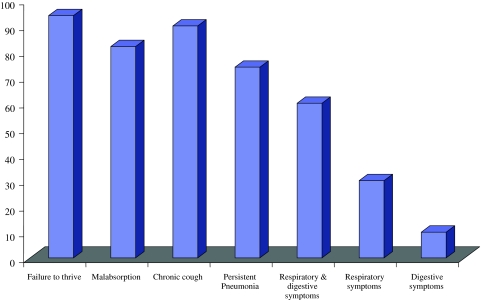
Clinical phenotype of CF patients [ 25 ]
Molecular Genetics of CFTR Gene
CF represents the first genetics disorder elucidated strictly by the process of positional cloning [ 11 ] succeeded in covering the CF region on long arm of chromosome 7 (7q31.2) by the chromosome walking and jumping. Screening of c DNA library constructed from cultured sweat gland cells proved that the CF gene was about 250 kb long [ 28 ]. Isolated overlapping c DNA clones from epithelial cell libraries with a genomic DNA segment containing apportion of putative CF gene. Transcript approximately 6,500 nucleotide in size were detectable. Riordan et al. [ 28 ] identified 24 exons in CF gene. The predicted protein has 1,480 amino acid with molecular mass of 1,68,138 Da. To avoid confusion with previously named CF antigen [ 28 ] referred to the protein as cystic fibrosis transmembrane conductance regulator gene (CFTR).
Structure of CFTR
The CFTR is a unique member of the ABC transporter family that forms a novel Chloride channel. After identification of the primary amino acid sequence of CFTR, Riordan et al. proposed a structure (Fig. 1 ) composed of two motifs, each containing a membrane spanning domain (MSD) that is composed of six transmembrane segments and a nucleotide binding domain (NBD) that contains sequence predicted to interact with ATP [ 28 , 29 ]. In CFTR, the two MSD–NBD motifs are linked by a unique domain called the R (regulatory domain) that contains multiple consequence phosphorylation sites and many charged amino acids. The carboxyl terminal (consisting of threonine, arginine, and leucine (TRL) of CFTR is anchored through a PDZ-type–binding interaction with the cytoskeleton and is kept in close approximation (Fig. 2 ) to a number of important proteins [ 30 ].
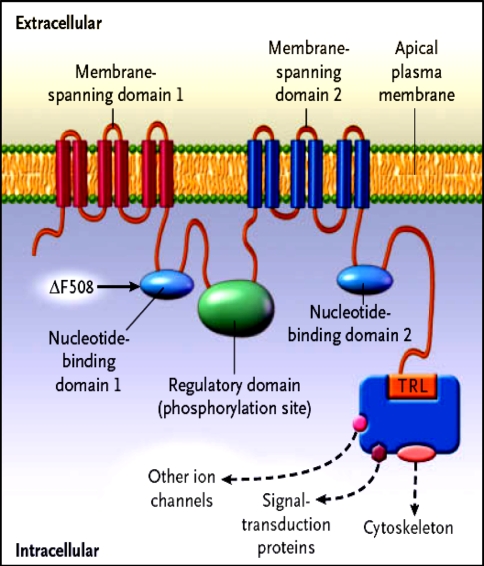
Hypothesized structure of CFTR showing proposed domain structure of CFTR protein. MSD membrane spanning domain, NBD nucleotide binding domain, R regulatory domain, PKA cAMP dependent protein kinase
Spectrum of CFTR Mutation
CF causing mutations have existed for more than 50,000 years [ 31 ]. To date more than 1,300 different alleles have been reported as proven or putative disease causing mutations to the Cystic Fibrosis Genetic Analysis Consortium (CFGAC). http://www.genet.sickkids.on.ca/cftr/.Worldwide there are 24 relatively common mutations identified in more than 50 chromosomes. The most common mutant allele is the delta F508 mutation which is a three-nucleotide deletion of a phenylalanine residue [ 32 ]. It is responsible for approximately two thirds (66%) of all CF chromosomes; however there is a great mutational heterogeneity in the remaining one-third of all alleles depending on populations and geographical locations [ 33 ].
Frequency of Delta F508 Mutation in Indian CF Population
The most common mutation in CF is the deletion of a phenylalanine residue at position 508 (delF508) in NBD1, accounting for about 66% of all CF alleles worldwide (CFGAC, http://www.genet.sickkids.on.ca/cftr/ ). However, it is not the predominant mutation in Asian CF patients [ 34 ]. While the delta F508 mutation is very common in number of different populations, its relative frequency varies for example between the north to south of Europe (delta F508 accounts for 90% of CF chromosomes in Denmark, and 30% in Turkey (Cystic Fibrosis Genetic Analysis Consortium, 1994). Schwarz et al. reported six affected Pakistani children, of whom three were homozygous for the delta F508 mutation [ 35 ].
By Kabra et al. [ 14 , 36 , 37 ], CF patients in India have been little studied. In the present investigation, we document that delta F508 mutation represents only 24% of the analyzed CF alleles. Comparison of delta F508 allele frequency with that reported from the Western countries shows that the Indian group has low percentage of the delta F508 frequency. A review of all genotyped South Asian patients, shows that delta F508 was identified in 19–44% of CF alleles [ 4 , 14 , 35 , 36 , 38 – 41 ], considerably lower than the reported frequency of 66% in the worldwide CF population. The frequency of delta F508 mutation in Indian children is reported to be between 19 and 44% [ 14 , 36 , 37 ]. In a report of 120 Indian children with CF [ 36 ], the delta F508 mutation was identified in 45 chromosomes (19%) out of the 240 tested, 19 patients (16%) were homozygous and 7 (6%) were heterozygous for the mutation. The frequency of delta F508 mutation was higher in patients whose parental origins were from Pakistan. Out of 23 patients whose origin was traced to Pakistan, 13 (56%) were positive for the delta F508 mutation. The figure for patients originating from other parts of India was 13 (13%) out of 97% patients. The spectrum of mutations other than delta F508 in Indian patients is highly variable and some rare and new mutations have been observed [ 25 , 36 ].
In our study it is documented delta F508 as the most common CFTR gene mutation in Indian population [ 12 , 25 ]. This observation supports the hypothesis that immigration patterns between US, Europe and the Indian subcontinent are contributing to the evolution of CF in the Indian subcontinent.
Spectrum of Non-Delta F508 CFTR Mutations in Indian CF Population
The striking characteristic of CF alleles in Indian population was the lack of common CF mutations in comparison with other Mediterranean and European population [ 31 ]. Apart from delta F508 mutation, twenty one other mutations were identified of which 13 were already known and 8 new mutations were characterized (Table 2 ). 25 mutation panel were detected in our population at a combined frequency of (10%). The other seven known but rare mutations (1161delC, Y517C, V520F, S549N, Y1381H, L218X and 1525-1G-A) were identified at a combined frequency of (17%), and eight new mutations (3986delC, 1792InsA, L69H, S158N, Q493L, I530L, E1329Q and 876-8del4) identified in our CF population represented (15%) of the total CF alleles analyzed. Substitution mutation, S549N and splice site mutation, 1525-1G-A were the two most common mutations identified with a frequency of (4%) followed by another splice site mutation 3849+10kbC-T (3%). However, we failed to identify CFTR gene mutations in 34% of the CF alleles in our study.
CFTR mutation identified in Indian population with classical CF [ 25 ]
U unidentified
In contrast, Kabra et al. characterized ten mutations on 46% of the chromosomes by screening 16 of 27 CFTR exons. Less number exons screened is probably the reason for non-conformity [ 36 ]. Shastri and Kabra reported 1161delC, 3849+10kbC-T and S549 N as other most common mutations in Indian population [ 26 ]. It is in accordance with our study. G542X, second most common mutation (5%) in Hispanic Caucasians [ 42 ], was not found in our population. W1282X and 621+1G-T, that occur with a frequency greater than 1% in Non-Hispanic Caucasians [ 42 ] were also not detected.
It reinforces our hypothesis that spectrum of mutations in India is different. Thus, it is emphasized that mutations specific for Indian population should be included in the panel for the genetic diagnosis of CF.
CFTR Dysfunction and Male Infertility Due to Obstructive Azoospermia
CF and obstructive azoospermia causing male infertility due to bilateral/unilateral absence of vas deferens/absence of seminal vesicles are such different phenotypes that they appear to be distinct clinical entities. CF is a life threatening disorder that is usually diagnosed by pediatricians during the first few years of life because of gastrointestinal and pulmonary manifestations and failure to thrive. Obstructive azoospermia is diagnosed by urologists in adult males presenting with infertility; some of these patients have additional renal malformations, which is not a symptom of CF. However, despite of these differences, there exists a genetic commonality for both diseases in the majority of patients. Infertility in males, which is generally accepted as an almost inevitable component of cystic fibrosis (CF), results from congenital absence of vas deferens in about 95% of male patients [ 43 , 44 ]. However, CBAVD may also occur in the absence of cystic fibrosis [ 24 ]. The question of whether obstructive azoospermia and cystic fibrosis are the “extreme” of one disease because such patients can be offered opportunity to have children. From this point of view, we searched for mutations in CFTR gene of obstructive azoospermia cases and their female partners.
In total, among 50 cases of genital CF (obstructive azoospermia) were studied by Prasad and coworkers, number of CBAVD and CUAD were 40 and 10 subjects respectively [ 25 ]. CFTR gene mutations were identified in 48 subjects. The mechanism by which the vas deferens is affected in cystic fibrosis is that the embryonic vas deferens needs an environment with electrolytes and fluid balance for potency. This balance is usually regulated by the CFTR present on vassal epithelial cells. However, in cystic fibrosis the function of this protein is defective and this balance is not attained, resulting in obliteration of vas deferens [ 45 ]. The seminal vesicles, body of epididymis are also affected but the testicular efferent ducts tend to be spared and some may be dilated [ 46 ].
A total of 12 mutations were identified on 48 of 100 alleles, as listed in Table 3 . Two mutations were found to be very common: first, the IVS8-T5 allele was observed on 25 chromosomes; thus confirming the association of this splice site variant with CAVD in Indian patients, second the F508del mutation was detected in 11 chromosomes. In the CAVD patients with normal renal development, the initial screening identified one extra, R117H mutation in three chromosomes. SSCP analysis performed in patients with only one or no mutation revealed nine further mutations on one allele each including seven new sequence alterations: L69H, F87I, G126S, F157C, E543A, Y852F and D1270E (Table 3 ). Other identified mutations R117H, 3120+1>G-A and P1021S have been described previously in studies of patients with CAVD. None of the other screened mutations were identified in our population.
CFTR mutations identified and characterized in the Indian patients with CAVD [ 12 ]
We documented NBD1 and NBD2 as the hotspot identified in the CFTR protein in Indian CF population, whereas the regions known to alter chloride permeability (transmembrane regions) and delta F508 mutation in NBD1 are the hot spot for mutation identification in our genital form of CF cases (obstructive azoospermia).
CF was thought to be extremely rare in India. However a review of all the reports from Indian subcontinent suggests that CF is probably far more common in people of India/Indian origin than previously thought but is under diagnosed or missed in the majority of cases. Our demographic and mutation data together indicate that 6% of patients were born to consanguineous couples. Therefore, carrier frequency is expected to be high among Indian. The carrier frequency of delta f508 in the Indian population was calculated to be 1 in 238 [ 14 ].
High genetic heterogeneity in our population accompanies a much higher carrier frequency than expected. The precise incidence of CF among Indians is unknown. As a result of the widespread belief that CF does not occur in Indians, the disease is rarely suspected and even if it is suspected the diagnosis is not confirmed due to the poor availability of facilities for diagnosis. The proper availability of mutation analysis, searching for mutations appropriate for the population or whole gene sequencing may help in better characterization of CFTR gene mutation in Indian population.
- 1. Prasad R, Marwaha RK, Kaur G, Walia BNS. Spectrum of biochemical genetic diseases in North India. Med Sci Res. 1998;26:455–6.
- 2. Bhakoo ON, Kumar R, Walia BNS. Mucoviscidosis of the lung. Indian Pediatr. 1968;35:183–185. doi: 10.1007/BF02808629. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Goodchild MC, Hansley J, Rushton DI, Gaze H. Cystic fibrosis in three Pakistani children. Arch Dis Child. 1974;49:739–741. doi: 10.1136/adc.49.9.739. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Powers CA, Potter EM, Wessel HU, Lloyd-Still JD. Cystic fibrosis in Asian Indians. Arch Pediatr Adolesc Med. 1996;150:554–555. doi: 10.1001/archpedi.1996.02170300108024. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study. Am J Dis Child. 1938;56:344–399. [ Google Scholar ]
- 6. Farber S. Some organic digestive disturbances in early life. J Mich Med Soc. 1945;44:587–594. [ Google Scholar ]
- 7. Di Sant’Agnese PA, Darling RC, Perera GA, Shea E. Abnormal electrolytic composition of sweat in cystic fibrosis of the pancreas. Clinical significance and relationship of the disease. Pediatrics. 1953;12:549–563. [ PubMed ] [ Google Scholar ]
- 8. Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Tsui LC, Buchwald M, Barker D, Braman JC, Knowlton R, Schumm JW, et al. Cystic fibrosis locus defined by a genetically linked polymorphic DNA marker. Science. 1985;230:1054–1057. doi: 10.1126/science.2997931. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Estivill X, Scambler PJ, Wainwright BJ, Hawley K, Frederick P, Schwartz M, et al. Patterns of polymorphism and linkage disequilibrium for cystic fibrosis. Genomics. 1987;1:257–263. doi: 10.1016/0888-7543(87)90052-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Sharma N, Acharya N, Singh SK, Singh M, Sharma U, Prasad R. Hetrogenous spectrum of CFTR gene mutation in Indian patients with congenital absence of vas deferens. Hum Reprod. 2009;1:1–8. doi: 10.1093/humrep/den500. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Spencer DA, Venkataraman M, Higgins S, Stevenson K, Weller PH. Cystic fibrosis in children from ethnic minorities in the West Midlands. Respir Med. 1994;88:671–675. doi: 10.1016/S0954-6111(05)80065-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Kabra M, Ghosh M, Kabra SK, Khanna A, Verma IC. Delta F508 molecular mutation in Indian children with cystic fibrosis. Indian J Med Res. 1996;104:355–358. [ PubMed ] [ Google Scholar ]
- 15. Kapoor V, Shastri SS, Kabra M, Kabra SS, Ramachandran V, Arora S, et al. Carrier frequency of F508del mutation of cystic fibrosis in Indian population. J Cyst Fibros. 2005;23:1631–1652. doi: 10.1016/j.jcf.2005.10.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Dequeker E, Cuppens H, Dodge J, Estivill X, Goossens M, Pignatti PR, et al. Recommendations for quality improvement in genetic testing for cystic fibrosis. European concerted action on cystic fibrosis. Eur J Hum Genet. 2000;8:S2–S24. doi: 10.1038/sj.ejhg.5200487. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Holsclaw DS, Perlmutter AD, Jockin H, Shwachman H. Genital abnormalities in male patients with cystic fibrosis. J Urol. 1971;106:568–574. doi: 10.1016/s0022-5347(17)61343-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Costes B, Girodon E, Ghanem N, Flori E, Jardin A, Soufir JC, et al. Frequent occurrence of the CFTR intron 8 (TG)n5T allele in men with congenital bilateral absence of vas deferens. Eur J Hum Genet. 1995;3:285–293. doi: 10.1159/000472312. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Dork T, Dworniczak B, Aulehla-Scholz C, Wieczorek D, Bohm I, Mayerova A, et al. Distinct spectrum of CFTR mutations in congenital absence of vas deferens. Hum Genet. 1997;100:365–377. doi: 10.1007/s004390050518. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Casals T, Nunes V, Palacios A, Gimenez J, Gaona A, Ibanez N, et al. Cystic fibrosis in Spain: high frequency of mutation G542X in the Mediterranean coastal area. Hum Genet. 1993;91:66–70. doi: 10.1007/BF00230225. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Grangeia A, Sá R, Carvalho F, Martin J, Girodon E, Silva J, et al. Molecular characterization of the cystic fibrosis transmembrane conductance regulator gene in congenital absence of the vas deferens. Genet Med. 2007;9:163–172. doi: 10.1097/GIM.0b013e3180318aaf. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Chillon M, Palacio A, Nunes V, Casals T, Gimenez J, Estivill X. Identification of a frameshift mutation (1609delCA) in exon 10 of the CFTR gene in seven Spanish cystic fibrosis patients. Hum Mutat. 1992;1:75–76. doi: 10.1002/humu.1380010113. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Casals T, Bassas L, Ruiz-Romero J, Chillón M, Giménez J, Ramos MD, et al. Extensive analysis of 40 infertile patients with congenital absence of the vas deferens: in 50% of cases only one CFTR allele could be detected. Hum Genet. 1995;95:205–211. doi: 10.1007/BF00209403. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Augarten A, Yahav Y, Keremn BS, Halle D, Laufer J, Szeinberg A, et al. Congenital bilateral absence of vas deferens in the absence of cystic fibrosis. Lancet. 1994;344:1473–1474. doi: 10.1016/S0140-6736(94)90292-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Sharma N, Singh M, Kaur G, Thapa BR, Prasad R. Identification and characterization of CFTR gene mutation in Indian CF patients. Ann Hum Genet. 2009;76:26–33. doi: 10.1111/j.1469-1809.2008.00477.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Shastri SS, Kabra SS. Mutation spectrum in India. In: Respicon 2006. Pre conference workshop on cysic fibrosis and fourth CF conference of India. Organised by Cystic Fibrosis Working Group, Respiratory Chapter, Indian Academy of Pediatrics, Nov 23, 2006.
- 27. Sharma N, Singh M, Acharya N, Singh SK, Thapa BR, Kaur G, Prasad R. Implication of cystic fibrosis transmembrane conductance regulator gene in infertile family members of Indian CF patients. Biochem Genet. 2008;46:847–56. [ DOI ] [ PubMed ]
- 28. Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, et al. Structural model of ATP-binding protein associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, et al. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Morral N, Bertanpetit J, Estivill X, Nunes V, Casals T, Gimenez J, et al. The origin of the major cystic fibrosis mutation (delta F508) in European populations. Nat Genet. 1994;7:169–175. doi: 10.1038/ng0694-169. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Estivill X, Bancells C, Ramos C, Biomed CF. Geographic distribution and regional origin of 272 cystic fibrosis mutations in European populations. Hum Mutat. 1997;10:135–154. doi: 10.1002/(SICI)1098-1004(1997)10:2<135::AID-HUMU6>3.0.CO;2-J. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Yamashiro Y, Shimizu T, Oguchi S, Shioya T, Nagata S, Ohtsura Y, et al. The estimated incidence of cystic fibrosis in Japan. J Pediatr Gastroenterol Nutr. 1997;24:544–554. doi: 10.1097/00005176-199705000-00010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Schwarz MJ, Super M, Wallis C, Beighton P, Newton C, Heptinstall LE, et al. Delta 508 testing of the DNA bank of the royal Manchester Children’s Hospital. Hum Genet. 1990;85:428–430. doi: 10.1007/BF02428298. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Kabra M, Kabra SK, Ghosh M, Khanna A, Arora S, Menon PS, et al. Is the spectrum of mutations in Indian patients with cystic fibrosis different? Am J Med Genet. 2000;93:161–163. doi: 10.1002/1096-8628(20000717)93:2<161::AID-AJMG15>3.0.CO;2-L. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Kabra SK, Kabra M, Lodha R, Shastri S, Ghosh M, Pandey RM, et al. Clinical profile and frequency of delta F508 mutation in Indian children with cystic fibrosis. Indian Pediatr. 2003;40:612–619. [ PubMed ] [ Google Scholar ]
- 38. Bowlers IM, Estelin EJ, Littlewood JM. Cystic fibrosis in Asians. Arch Dis Child. 1993;68:120–122. doi: 10.1136/adc.68.1.120. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Curtis A, Richardson RJ, Boohene J, Jackson A, Nelson R, Bhattacharya SS. Absence of cystic fibrosis mutations in a large Asian population sample and occurrence of a homozygous S549N mutation in an inbred Pakistani family. J Med Genet. 1993;30:164–166. doi: 10.1136/jmg.30.2.164. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Bhutta ZA, Moattar T, Shah U. Genetic analysis of cystic fibrosis in Pakistan: a preliminary report. J Pakistan Med Assoc. 2000;50:217–219. [ PubMed ] [ Google Scholar ]
- 41. Mei-Zahav M, Durie P, Zielenski J, Solomon M, Tullis E, Tsui LC, et al. The prevalence and clinical characteristics of cystic fibrosis in South Asian Canadian immigrants. Arch Dis Child. 2005;90:675–679. doi: 10.1136/adc.2003.042614. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Watson MS, Cutting GR, Desnick RJ, Driscoll DA, Klinger K, Mennuti M. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genet Med. 2004;6:387–391. doi: 10.1097/01.GIM.0000139506.11694.7C. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Kaplan E, Swachman H, Permutter AD, Rule A, Khaw KT, Holsclaw DS. Reproductive failure in males with cystic fibrosis. N Engl J Med. 1968;279:65–69. doi: 10.1056/NEJM196807112790203. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Jequier AM, Ansell ID, Bullimore NJ. Congenital absence of the vas deferens presenting with infertility. J Androl. 1985;6:15–19. [ PubMed ] [ Google Scholar ]
- 45. Oates RD, Amos JA. Congenital bilateral absence of vas deferens and cystic fibrosis: a genetic commonality. World J Urol. 1993;11:82–88. doi: 10.1007/BF00182034. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Landing BH, Wells TR, Wang CI. Abnormality of the epididymis and vas deferens in cystic fibrosis. Arch Pathol. 1969;88:569–580. [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (272.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
COMMENTS
In the largest case series of cystic fibrosis from India, reported from our center [29], majority of the clinical and laboratory manifestations at presentation were similar to that described in Western series and included recurrent or persistent pneumonia, malabsorption, failure to thrive, rectal prolapse, salt craving, salty taste on kissing ...
Dec 1, 2015 · PDF | On Dec 1, 2015, Anirban Mandal and others published Cystic Fibrosis in India: Past, Present and Future | Find, read and cite all the research you need on ResearchGate ... a case-control ...
Jun 15, 2024 · Cystic fibrosis (CF) is a life-threatening monogenic disease affecting thousands of people worldwide. Cystic fibrosis transmembrane conductance regulator (CFTR) is an ion channel that facilitates transportation of water and salts across epithelial cell membranes through the conductance of Cl− and other anions. A dysfunctional CFTR due to abnormalities in the cftr gene causes CF, which is ...
Case Report Volume 3 Issue 5 -March 2017 DOI: 10.19080/AJPN.2017.03.555681 Acad J Ped Neonatol ... Cystic Fibrosis in India- Need for Study Pramila Menon*, Krishnadas ...
Apr 30, 2022 · Determination of sweat electrolyte is considered as optimal diagnostic method. Results of previous studies have shown that cystic fibrosis increase the sodium and chloride concentrations. Mutations like ΔF508, G542X, R553X, N130K and 621+1 (G →T) are most common in CF patients in India. Among them ΔF508 is most severe and predominant mutation.
Aug 1, 2012 · A case- series of 14 children (9 males and 5 females) with cystic fibrosis is presented who came with persistent/recurrent pneumonia. Most children had infantile onset of symptoms (mean age 4.6±4 ...
100% and 85% suspect cystic fibrosis respectively. 90% do not have facilities for diagnosis of cystic fibrosis like sweat chloride test and 87.5% felt absence of facilities makes the diagnosis of cystic fibrosis difficult. 90% agreed that the education of pediatricians about the disease, can improve the quality of life and survival in CF.
Apr 30, 2022 · Results of previous studies have shown that cystic fibrosis increase the sodium and chloride concentrations. Mutations like ΔF508, G542X, R553X, N130K and 621+1 (G →T) are most common in CF ...
Dec 31, 2015 · A higher carrier frequency for genetic deafness, cystic fibrosis and Pompe disease was unexpected, and contrary to the generally held view about their prevalence in Asian Indians, to suggest that population-based carrier screening panels for India would differ from those in the West. Expand
Before seventies, CF was considered as non existent in India. First case of CF disease was reported in 1968 from PGIMER, Chandigarh . Later, suggested incidence of cystic fibrosis (CF) in Asian migrants (Indians and Pakistanis) in the United Kingdom was reported about 1 in 10,800 to 12,000 [3, 4]. Nevertheless, there have been many publications ...