
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

Potassium Permanganate Volcano

The potassium permanganate volcano is a classic chemistry demonstration that produces smoke, colored flames and sparks, a glow resembling lava, and colored ash. This type of chemical volcano captivates students while illustrating principles of redox reactions, oxidation , exothermic reactions , the effect of surface area on reaction rate, decomposition reactions, and the flame test. The demonstration is ideal for middle school and high school chemistry students.
Chemical Volcano Materials
The volcano only requires two chemicals and a few basic materials. You can customize the size of the reaction based on the amount of chemicals you want to use and the effect you’re seeking.
- 2-3 grams of potassium permanganate crystals (KMnO 4 )
- 1 milliliter glycerol in a test tube (also known as glycerin or glycerine)
- Porcelain dish
Note: Fine crystals of potassium permanganate work better than large crystals. You may wish to perform the demonstration with equal masses of large and small crystals (made by grinding large crystals with a mortar and pestle) to illustrate the effect of surface area on rate of reaction. Expect a delayed reaction if larger crystals are used.
Note: Fresh glycerol works better than “old” glycerol. Possibly this is because glycerol absorbs water from air. Gently warming old glycerol before use improve may drive off any water and improve the display.
- Place the potassium permanganate crystals in a mound in the dish. If you like, you can make a shape resembling the cinder cone of a volcano. You can improve the display by pushing a small indentation into the top of the mound, resembling a volcanic caldera.
- Pour the glycerol on top of the potassium permanganate and immediately move back. If you are studying reaction kinetics, start a stopwatch immediately after moistening the potassium permanganate.
- Very fine crystals react almost immediately, while coarse crystals may take 30 seconds. Initially, expect the mixture to steam, smoke, and crackle. Next, it will burst into flame. The flame color will display the characteristic pinkish-purple of the flame test for potassium . Finally, the flames will die back, leaving an orange glow and black, green, or brown ash.
- If your discussion includes redox reactions and oxidation states, you may allow the residue to cool and then dissolve the ash in water. The resulting green solution indicates the presence of Mn(VI), with solid brown manganese(IV) oxide. Potassium permanganate contains Mn(VII), so the colors of the products indicate manganese was reduced. Alternatively, you can add potassium permanganate to a strong base solution (e.g., sodium hydroxide solution) to yield the purple color and the ash from the volcano to the strong base solution to yield a yellow-green color, thus demonstrating the reactant and product are different.
Note: If the mixture fails to “erupt” it should be discarded by washing it down the drain with a large volume of water. Do not discard the mixture in a trash can as it may eventually ignite at some later time!
How the Potassium Permanganate Volcano Works
Potassium permanganate (KMnO 4 ) is a strong oxidizer, while glycerol (C 3 H 5 (OH) 3 ) is an alcohol that is readily oxidized. The reaction between the two chemicals produces steam, carbon dioxide gas, and manganese oxide according to the following reaction: 14KMnO 4 (s) + 4C 3 H 5 (OH) 3 (l) → 7K 2 CO 3 (s) + 7Mn 2 O 3 (s) + 5CO 2 (g) + 16H 2 O(g)
Like other chemical fire demonstrations, the reaction is spontaneous. As it releases heat, it is exothermic. The purple flame color results from potassium electrons absorbing energy, becoming excited, and releasing light as they return to a lower energy state.
Safety Information
Potassium permanganate and glycerol are considered to be safe chemicals for chemistry demonstrations. Glycerol is safe enough that it is used as a food additive. Potassium permanganate is even used to treat skin condition and to remove the rotten egg smell from hydrogen sulfide in well water. It does not generate toxic by-products. However, potassium permanganate is a strong oxidizer and can stain skin pink to purple. If contact occurs, wash the affected area with water. Also, the reaction releases smoke, heat, and flames. For optimal safety, the reaction should be kept small, using no more the 10 grams of potassium permanganate. The chemical volcano demonstration should be performed wearing gloves, protective clothing, and safety goggles. After the project is complete, the residue may be washed down the drain with water.
- Chambers, Michael. “ ChemIDplus – 7722-64-7 – VZJVWSHVAAUDKD-UHFFFAOYSA-N – Potassium permanganate [USP:JAN] – Similar structures search, synonyms, formulas, resource links, and other chemical information .”
- Lister, T.; O’Driscoll, C.; Reed, N. (1995). Classic Chemistry Demonstrations . London, UK: Royal Society of Chemistry. pp. 65–70. ISBN 978-1-87034-338-1.
- Shakhashiri, B. Z. (1983). Chemical Demonstrations Volume 1: A Handbook for Teachers of Chemistry . University of Wisconsin Press: Madison, Wisconsin: University of Wisconsin Press. pp. 83–84. ISBN 9780299088903.
- Summerlin, L. R. (1988). Chemical Demonstrations : A Sourcebook for Teachers (Vol. 1, 2nd ed.). Washington, DC: American Chemical Society. p. 122. ISBN 978-0841215351.
Related Posts

How to Make an Awesome Volcano Science Project
This post may contain affiliate links.

Want to know how to make a volcano with your kids? Making a volcano that erupts is one of those good old classic science projects that kids just love doing! I am surprised that I don’t have this one on my site yet, because it’s a favorite! Also, grab your free printable Volcano Diagram Worksheets, too!

My husband found an old book this past week for my son called 101 Cool Science Experiments by Glen Singleton. We flipped through the book together marking all of the pages of the experiments that we want to try. He is a little bit of a science fanatic like me. We both get excited about new experiments! He was most excited to try making a volcano!
It’s funny, so many of my experiments were done with my two older kids and now we are really enjoying recreating them with the younger half of the family. This was a favorite of my oldest son snd now the younger kids had a blast with it! Get ready for mess.
Also check out out our Erupting Dinosaur Extinction Slime !
How to Make a Volcano
Watch it all here, or read on for the written instructions.
We made our volcano model with salt dough. Salt dough is super simple to make and can be air dried if you want to make it ahead and save it for a science fair project.
Salt Dough Recipe

6 cups of Flour
2 cups of Salt
2 cups of Water
2 Tbsp of Cooking Oil
Coloring (you can use food coloring or liquid water color)
Mix the best you can with a spoon, but you may need to just dig in with your hands. That’s what I had to do. This is a hard dough so you can mold it and make it stand up around the bottle for your volcano filling. You may need to add a little bit more water depending on your climate. I added 1/4-1/3 cup more after I got into it.
You may notice the gray swirls in our dough. We tried to color our dough with some black liquid watercolors , but it would have taken the whole bottle, so we gave up!
Building the Volcano

You will need a bottle in the center. You can use a soda bottle a water bottle or a glass bottle like we used. Whatever you have on hand that has a smaller opening on top should work just fine!
Place the bottle in the center of a large cookie sheet. Mold the salt dough into a volcano shape around the bottle making sure to leave an opening at the top to add your volcano’s “lava” filling.

This is tons of fun to shape it to look like an amazing volcano. My kids were creating paths for the lava to flow through.
How to Make a Volcano Erupt (With Baking Soda & Vinegar)
Now to make the eruption solution! Making the volcano erupt is fun, messy and is over pretty quick, so don’t blink! 😉
Ingredients to Make your Volcano Erupt:

Red Food Coloring- Or red liquid watercolors
A few drops of Dish Soap- about a Tablespoon
2 Tbsp Baking Soda
Mix the red coloring, water, and some dish soap together. We used about 2 cups of water, but you’ll just need to fill your bottle about 3/4 of the way full.

Put 2 Tbsp of baking soda into the bottle.
Pour in your vinegar and watch the eruption go! Now is the perfect time to teach about the chemical reaction between the baking soda and vinegar.

Can we do it again?! (That’s what your kids will say.)

I am loving that I captured their excited expressions!

Have you done this fun, classic experiment with your kids?


Volcano Variations:
Make a volcano erupt with smoke by adding dry ice! Check out my smoking dragon post for tips.
Make a thicker lava by trying elephant toothpaste in the bottle!
Volcano Diagram Worksheets
Extend the learning with these Printable Volcano Diagram worksheets! This printable set includes color and black and white diagrams with both labeled and unlabeled versions. You can use them for learning and for quizzing later! Download your Volcano Diagrams now!
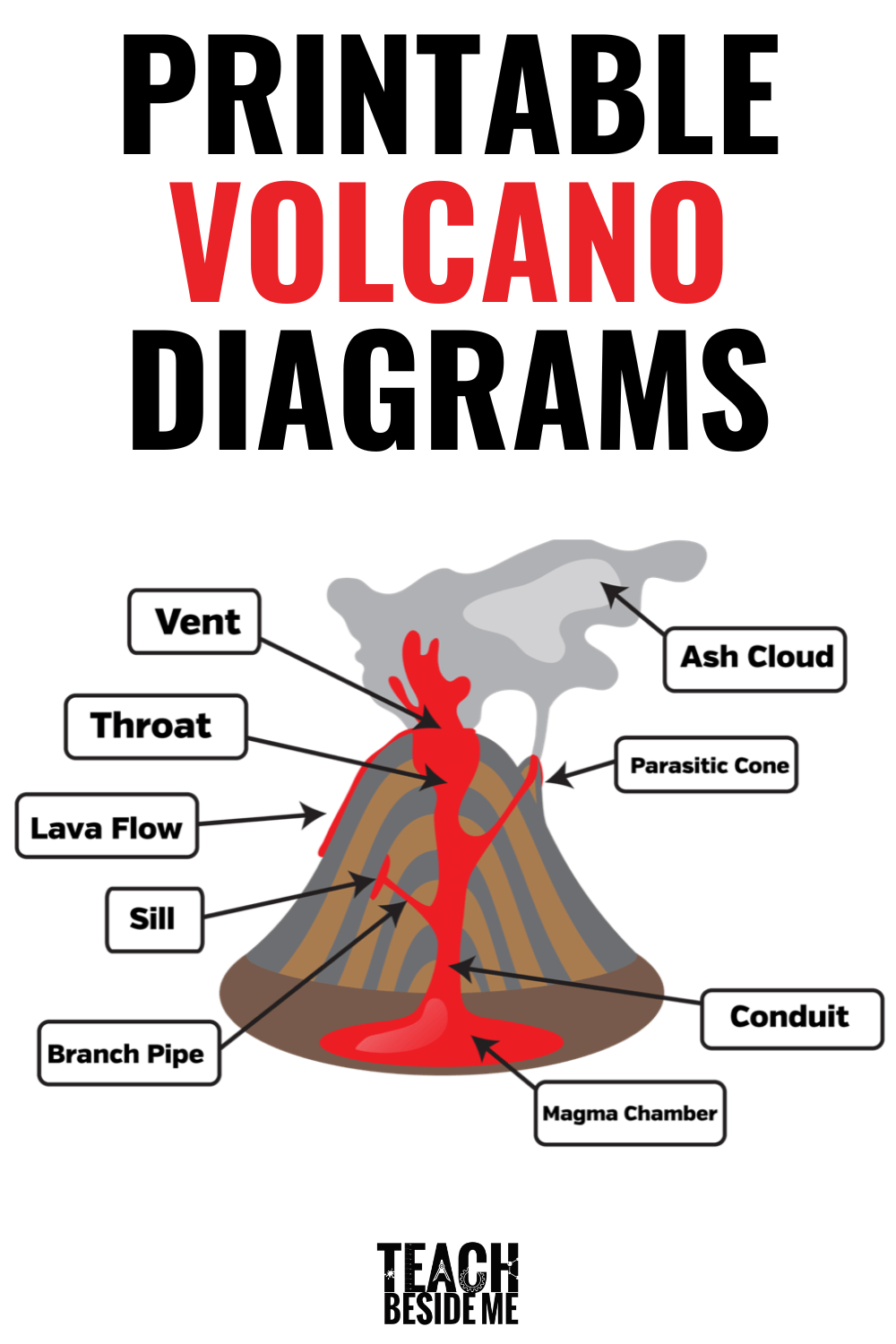
Learn about Real Volcanos and how they really erupt by reading some books on Volcanoes!
Volcanoes! Mountains for Fire by Eric Arnold is a great place to start.
We also like the Magic School Bus Blows its Top by Gail Herman
You may also enjoy: 30 More Baking Soda and Vinegar Experiments! It has some more fun ways to experiment with this chemical reaction.

Check out my Science Book! Science Art and Drawing Games for Kids

Former school teacher turned homeschool mom of 4 kids. Loves creating awesome hands-on creative learning ideas to make learning engaging and memorable for all kids!
Similar Posts

Homemade CD Spinner Toy

How to Make Pinwheels -With Templates

Science Art: Conductive Paint Circuits
Letter of the Week: Preschool Letter X Activities

Nature Science Experiments {Solar S’mores}

Glowing Invisible Ink Secret Messages
One comment.
I couldn’t find the instructions but some how i found out how to do it with now instructions
Leave a Reply Cancel reply
You must be logged in to post a comment.

IMAGES
VIDEO