LOADING... 0 %
- Staff picks
- Downloadable
- Collections
- Community members
- Sketchfab Masters
- Animals & Pets
- Architecture
- Art & Abstract
- Cars & Vehicles
- Characters & Creatures
- Cultural Heritage & History
- Electronics & Gadgets
- Fashion & Style
- Food & Drink
- Furniture & Home
- Nature & Plants
- News & Politics
- Places & Travel
- Science & Technology
- Sports & Fitness
- Weapons & Military
- Buy 3D models
- For business Sketchfab for Teams Augmented Reality 3D Viewer 3D eCommerce 3D Configurators Find a Partner Pricing Customer Stories

Rutherford's scattering experiment 3D Model
There are two animations. Select at bottom of model. This is the setup Rutherford has used. Radioactive material is used to emmit alpha particles. A beam of these alpha particles are directed to the gold foil in the middel. Most of the particle pass through the gold foil without being deflected. From this observation Rutherford concluded that most of the gold atoms must consist of empty space. The atoms’ mass is concentrated in the middel.
CC Attribution Creative Commons Attribution
- Science & technology 3D Models

- Why Does Water Expand When It Freezes
Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks
Who did the Gold Foil Experiment?
The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom . Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of Manchester between 1908 and 1913.
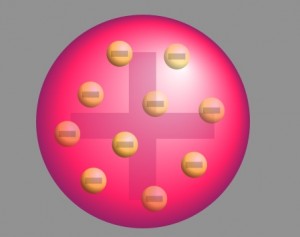
The prevalent atomic theory at the time of the research was the plum pudding model that was developed by Lord Kelvin and further improved by J.J. Thomson. According to the theory, an atom was a positively charged sphere with the electrons embedded in it like plums in a Christmas pudding.
With neutrons and protons yet to be discovered, the theory was derived following the classical Newtonian Physics. However, in the absence of experimental proof, this approach lacked proper acceptance by the scientific community.
What is the Gold Foil Experiment?
Description.
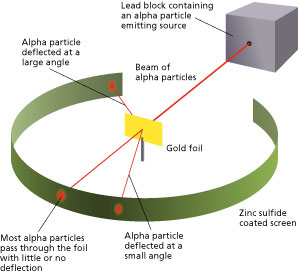
The method used by scientists included the following experimental steps and procedure. They bombarded a thin gold foil of thickness approximately 8.6 x 10 -6 cm with a beam of alpha particles in a vacuum. Alpha particles are positively charged particles with a mass of about four times that of a hydrogen atom and are found in radioactive natural substances. They used gold since it is highly malleable, producing sheets that can be only a few atoms thick, thereby ensuring smooth passage of the alpha particles. A circular screen coated with zinc sulfide surrounded the foil. Since the positively charged alpha particles possess mass and move very fast, it was hypothesized that they would penetrate the thin gold foil and land themselves on the screen, producing fluorescence in the part they struck.
Like the plum pudding model, since the positive charge of atoms was evenly distributed and too small as compared to that of the alpha particles, the deflection of the particulate matter was predicted to be less than a small fraction of a degree.
Observation
Though most of the alpha particles behaved as expected, there was a noticeable fraction of particles that got scattered by angles greater than 90 degrees. There were about 1 in every 2000 particles that got scattered by a full 180 degree, i.e., they retraced their path after hitting the gold foil.
Simulation of Rutherford’s Gold Foil Experiment Courtesy: University of Colorado Boulder
The unexpected outcome could have only one explanation – a highly concentrated positive charge at the center of an atom that caused an electrostatic repulsion of the particles strong enough to bounce them back to their source. The particles that got deflected by huge angles passed close to the said concentrated mass. Most of the particles moved undeviated as there was no obstruction to their path, proving that the majority of an atom is empty.
In addition to the above, Rutherford concluded that since the central core could deflect the dense alpha particles, it shows that almost the entire mass of the atom is concentrated there. Rutherford named it the “nucleus” after experimenting with various gases. He also used materials other than gold for the foil, though the gold foil version gained the most popularity.
He further went on to reject the plum pudding model and developed a new atomic structure called the planetary model. In this model, a vastly empty atom holds a tiny nucleus at the center surrounded by a cloud of electrons. As a result of his gold foil experiment, Rutherford’s atomic theory holds good even today.
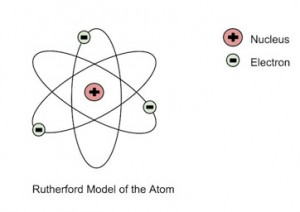
Rutherford’s Atomic Model
Rutherford’s Gold Foil Experiment Animation
- Rutherford demonstrated his experiment on bombarding thin gold foil with alpha particles contributed immensely to the atomic theory by proposing his nuclear atomic model.
- The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
- The significance and purpose of the gold foil experiment are still prevalent today. The discovery of the nucleus paved the way for further research, unraveling a list of unknown fundamental particles.
- Chemed.chem.purdue.edu
- Chem.libretexts.org
- Large.stanford.edu
- Radioa ctivity.eu.com
Article was last reviewed on Friday, February 3, 2023
Related articles

5 responses to “Gold Foil Experiment”
Super very much helpful to me,clear explanation about every act done by our Rutherford that is under different sub headings ,which is very much clear to ,to study .very much thanks to the science facts.com.thank u so much.
Good explanation,very helpful ,thank u ,so much
very clear and helpful, perfect for my science project!
Thank you for sharing the interactive program on the effects of the type of atom on the experiment! Looking forward to sharing this with my ninth graders!
Rutherford spearheaded with a team of scientist in his experiment of gold foil to capture the particles of the year 1911. It’s the beginning of explaining particles that float and are compacted . Rutherford discovered this atom through countless experiments which was the revolutionary discovery of the atomic nuclear . Rutherford name the atom as a positive charge and the the center is the nucleus.
Barack Hussein Obama
Mrs. Danize Obama
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
- Gases and liquids
- Structure of matter
- Atomic models
- Chemical bonds
- Structure of metals
- Ductility of metals
- Solidification of metals
- Steelmaking
- Iron-carbon phase diagram
- Heat treatment of steels
- Material testing
- Planetary gear
- Involute gear
- Cycloidal gear
- Temperature
- Kinetic theory of gases
- Thermodynamic processes in closed systems
- Thermodynamic processes in open systems
- Geometrical optics

Rutherford’s atomic model
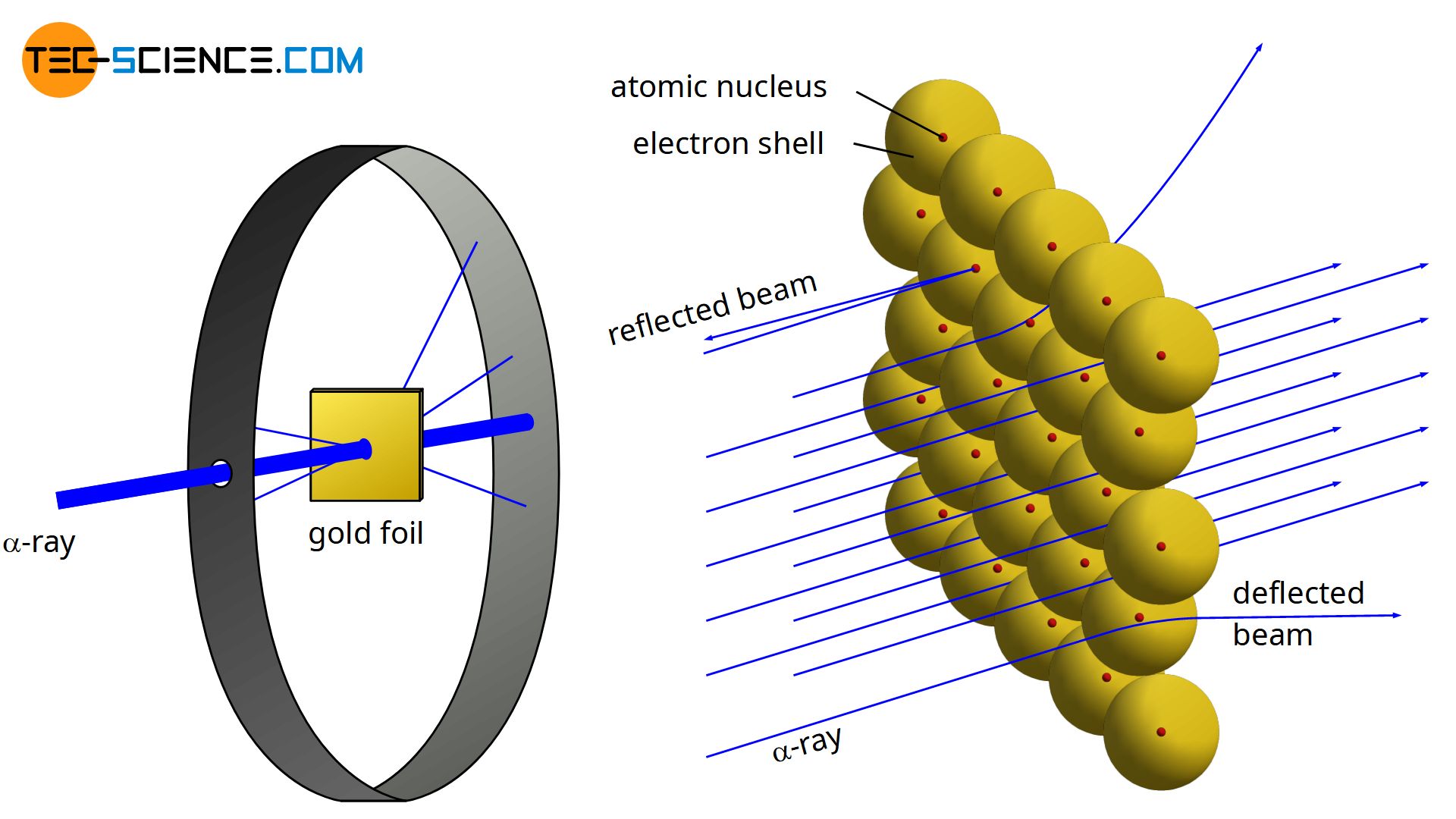
According to Rutherford’s atomic model, negatively charged electrons move around a positively charged atomic nucleus.
In 1910, the physicist Ernest Rutherford found that when a thin gold foil was bombarded with α-particles (twice positively charged helium nuclei with two neutrons \( ^4_2\text{He}^{2+} \)), only very few of these particles collided with the atomic nuclei of the gold atoms. Almost all α-particles traveled on a straight trajectory through the foil, while only a few were deflected.

Obviously, very few α-particles were close enough to the positive nucleus of the gold atoms that they could be deflected to a significant degree by the repulsive forces. In most cases, the α-particles traversed the gold foil at quite a distance from the respective atomic nuclei and were scarcely affected in their trajectory. This experiment concluded that the nucleus would have to be much smaller compared to the rest of the atom or rather to its atomic shell.
Today we know that the atomic nucleus has a diameter which is 10,000 to 100,000 times smaller than the atomic shell! If the atomic nucleus had the size of a dollar coin, the diameter of the atomic shell would amount to about 2 km!
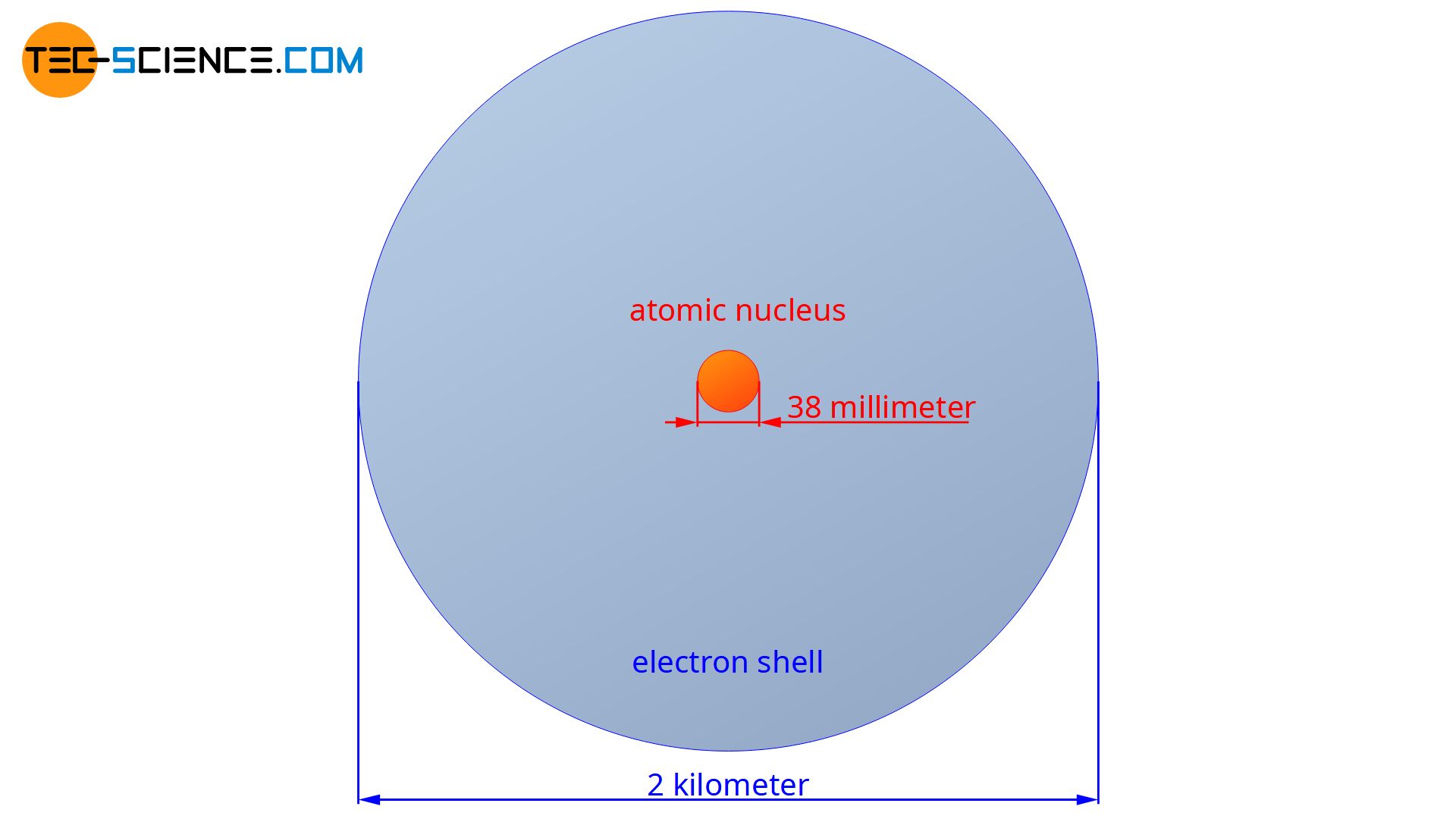
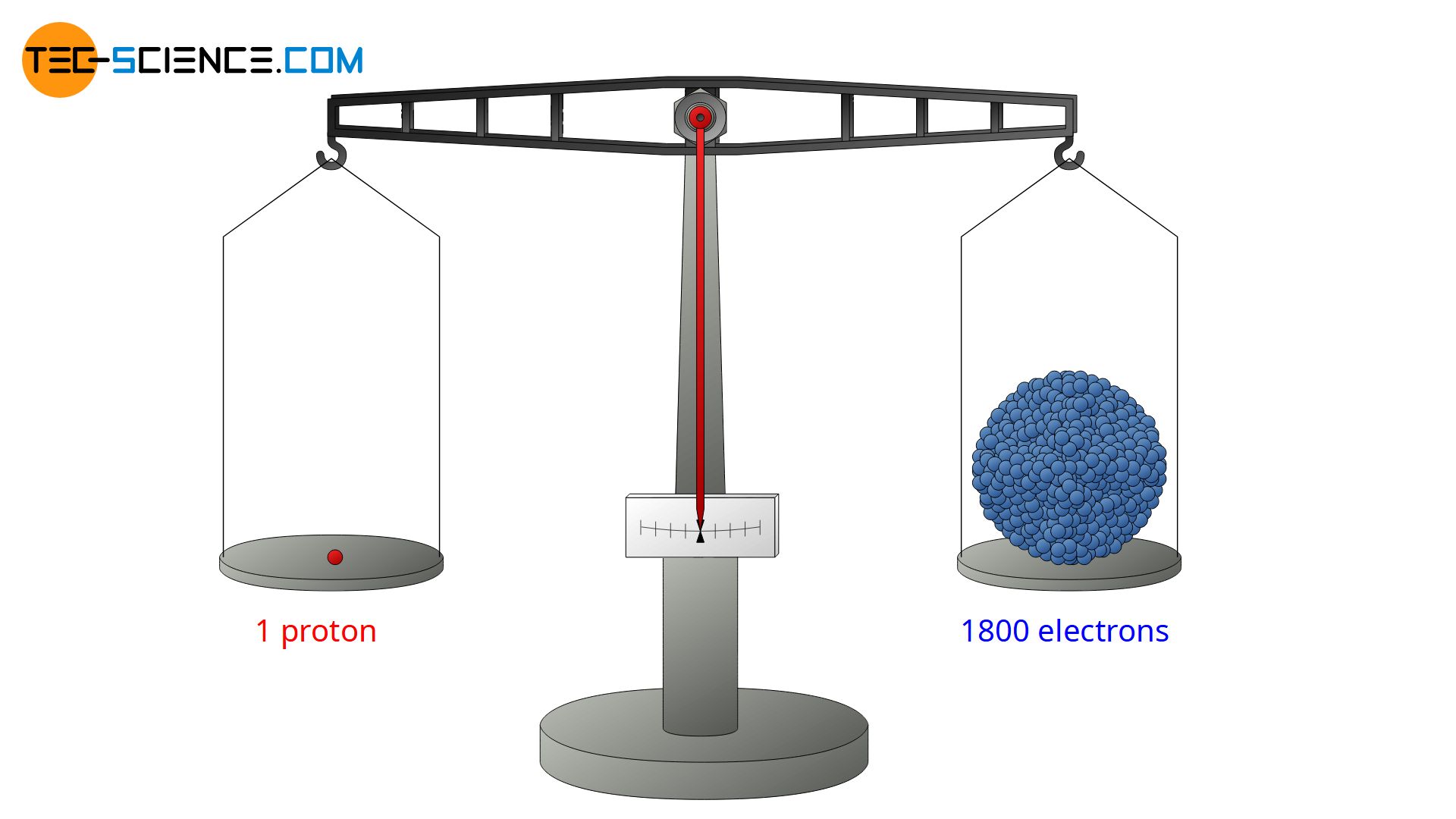
The gold foil experiment further showed that some α-particles were reflected back to the gold foil with almost no energy loss. They obviously had to hit something very massive and heavy (analogous to a tennis ball that hits a massive concrete wall and flies back at almost the same speed). From this, Rutherford concluded that nearly the entire mass of an atom must be concentrated in the nucleus to produce such a strong reflection effect. And indeed, nearly 99.9% of the total mass of an atom is contained in its nucleus. Only 0.1% of the mass is therefore attributable to the atomic shell. Today we know that a proton (as well as a neutron) has a mass about 1800 times as large as an electron.
These findings formed the basis for Rutherford’s atomic model ( Rutherford model ), whose quintessences are summarized below:
- an atom consists of an atomic nucleus and an atomic shell,
- the nucleus is positively charged and the atomic shell carries a negative charge,
- in the nucleus are positively charged protons (and neutrons),
- in the atomic shell are the negatively charged electrons,
- the nucleus is much smaller than the atomic shell and
- almost the entire mass of an atom is concentrated in its nucleus.
With the Rutherford model, the results of scattering experiments (such as those of the gold foil experiment) could be correctly explained. The basic mass and size ratios as well as the corresponding division into atomic nucleus and electron shell also reflect this atomic model.
For example, the question of why atoms can only be excited with certain energies can not be answered by this model. Or why atoms emit characteristic line spectra. Likewise, Rutherford’s atomic model gives no explanation why an atom is stable, because the circular motion of the electrons around the nucleus would actually lead to an energy dissipation. Accordingly, the electrons ought to fall into the nucleus after only a short time and no atom should therefore be stable!
Some of the weaknesses of Rutherford’s atomic model could be corrected by the physicist Niels Bohr in his model ( Bohr model ).
In principle, models (such as the atomic models or the particle model) never claim to give a complete explanation of reality. Models are always attempts to depict reality within certain limits and make it explainable.
The Rutherford model is not fundamentally “wrong” but has only limits of validity. Therefore, the Rutherford is not obsolete but it depends on the phenomena to be described and explained. To explain, for example, the gold foil experiment, the Rutherford model is completely sufficient; this does not require an unnecessarily complex quantum mechanical model.
Models are attempts to describe observable phenomena within certain validity limits.

RELATED ARTICLES MORE FROM AUTHOR
Bohr-Sommerfeld model
Bohr’s atomic model
- Legal notice
- Privacy Policy
KentChemistry HOME
Dalton's Model of the Atom / J.J. Thomson / Millikan's Oil Drop Experiment / Rutherford / Niels Bohr / DeBroglie / Heisenberg / Planck / Schrödinger / Chadwick
From MIT 3.091-Lec 3 Donald Sadoway 17:00 min
Earnest Rutherford- From New Zealand, one of 12 children born on a farm. Rutherford was a scholarship research student under J.J. Thomson at Cavendish Lab at Cambridge University. Did thesis research on the properties of charged particles. Identified alpha particle as a Helium nucleus (protons and neutrons, no electrons) and beta particle as an electron.
After this he worked at McGill University in Montreal, Canada. He worked on the origin of alpha particles (from the disintegration of elements) and won the Noble Prize .
He became a Professor of Physics at Victoria University in Manchester, UK.
The experiment to probe the structure of the atom performed by Hans Geiger (Geiger counter) and Ernest Marsden in 1909, under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester.
(Rutherford gets all the credit, while his graduate students did the work.)
The Experiment
A beam of alpha particles, generated by the radioactive decay of radium, was directed onto a sheet of very thin gold foil.
The gold foil was surrounded by a circular sheet of zinc sulfide (ZnS) which was used as a detector: The ZnS sheet would light up when hit with alpha particles.
The Results
"It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backward must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of the atom was concentrated in a minute nucleus. It was then that I had the idea of an atom with a minute massive center, carrying a charge." —Ernest Rutherford Adjustment to the Model of the Atom- Now with open space & positive nucleus J.J. Thomson Rutherford
Problems with the new model resulted in a strong negative reaction in the scientific community
Adjustment to the Model of the Atom- Now with open space & positive nucleus
What is the 'Gold Foil Experiment'? The Geiger-Marsden experiments explained
Physicists got their first look at the structure of the atomic nucleus.

J.J. Thomson model of the atom
Gold foil experiments, rutherford model of the atom.
- The real atomic model
Additional Resources
Bibliography.
The Geiger-Marsden experiment, also called the gold foil experiment or the α-particle scattering experiments, refers to a series of early-20th-century experiments that gave physicists their first view of the structure of the atomic nucleus and the physics underlying the everyday world. It was first proposed by Nobel Prize -winning physicist Ernest Rutherford.
As familiar as terms like electron, proton and neutron are to us now, in the early 1900s, scientists had very little concept of the fundamental particles that made up atoms .
In fact, until 1897, scientists believed that atoms had no internal structure and believed that they were an indivisible unit of matter. Even the label "atom" gives this impression, given that it's derived from the Greek word "atomos," meaning "indivisible."

But that year, University of Cambridge physicist Joseph John Thomson discovered the electron and disproved the concept of the atom being unsplittable, according to Britannica . Thomson found that metals emitted negatively charged particles when illuminated with high-frequency light.
His discovery of electrons also suggested that there were more elements to atomic structure. That's because matter is usually electrically neutral; so if atoms contain negatively charged particles, they must also contain a source of equivalent positive charge to balance out the negative charge.
By 1904, Thomson had suggested a "plum pudding model" of the atom in which an atom comprises a number of negatively charged electrons in a sphere of uniform positive charge, distributed like blueberries in a muffin.
The model had serious shortcomings, however — primarily the mysterious nature of this positively charged sphere. One scientist who was skeptical of this model of atoms was Rutherford, who won the Nobel Prize in chemistry for his 1899 discovery of a form of radioactive decay via α-particles — two protons and two neutrons bound together and identical to a helium -4 nucleus, even if the researchers of the time didn't know this.
Rutherford's Nobel-winning discovery of α particles formed the basis of the gold foil experiment, which cast doubt on the plum pudding model. His experiment would probe atomic structure with high-velocity α-particles emitted by a radioactive source. He initially handed off his investigation to two of his protégés, Ernest Marsden and Hans Geiger, according to Britannica .
Rutherford reasoned that if Thomson's plum pudding model was correct, then when an α-particle hit a thin foil of gold, the particle should pass through with only the tiniest of deflections. This is because α-particles are 7,000 times more massive than the electrons that presumably made up the interior of the atom.
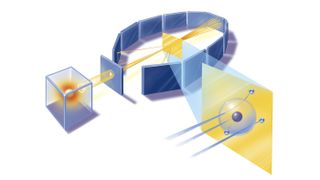
Marsden and Geiger conducted the experiments primarily at the Physical Laboratories of the University of Manchester in the U.K. between 1908 and 1913.
The duo used a radioactive source of α-particles facing a thin sheet of gold or platinum surrounded by fluorescent screens that glowed when struck by the deflected particles, thus allowing the scientists to measure the angle of deflection.
The research team calculated that if Thomson's model was correct, the maximum deflection should occur when the α-particle grazed an atom it encountered and thus experienced the maximum transverse electrostatic force. Even in this case, the plum pudding model predicted a maximum deflection angle of just 0.06 degrees.
Of course, an α-particle passing through an extremely thin gold foil would still encounter about 1,000 atoms, and thus its deflections would be essentially random. Even with this random scattering, the maximum angle of refraction if Thomson's model was correct would be just over half a degree. The chance of an α-particle being reflected back was just 1 in 10^1,000 (1 followed by a thousand zeroes).
Yet, when Geiger and Marsden conducted their eponymous experiment, they found that in about 2% of cases, the α-particle underwent large deflections. Even more shocking, around 1 in 10,000 α-particles were reflected directly back from the gold foil.
Rutherford explained just how extraordinary this result was, likening it to firing a 15-inch (38 centimeters) shell (projectile) at a sheet of tissue paper and having it bounce back at you, according to Britannica
Extraordinary though they were, the results of the Geiger-Marsden experiments did not immediately cause a sensation in the physics community. Initially, the data were unnoticed or even ignored, according to the book "Quantum Physics: An Introduction" by J. Manners.
The results did have a profound effect on Rutherford, however, who in 1910 set about determining a model of atomic structure that would supersede Thomson's plum pudding model, Manners wrote in his book.
The Rutherford model of the atom, put forward in 1911, proposed a nucleus, where the majority of the particle's mass was concentrated, according to Britannica . Surrounding this tiny central core were electrons, and the distance at which they orbited determined the size of the atom. The model suggested that most of the atom was empty space.
When the α-particle approaches within 10^-13 meters of the compact nucleus of Rutherford's atomic model, it experiences a repulsive force around a million times more powerful than it would experience in the plum pudding model. This explains the large-angle scatterings seen in the Geiger-Marsden experiments.
Later Geiger-Marsden experiments were also instrumental; the 1913 tests helped determine the upper limits of the size of an atomic nucleus. These experiments revealed that the angle of scattering of the α-particle was proportional to the square of the charge of the atomic nucleus, or Z, according to the book "Quantum Physics of Matter," published in 2000 and edited by Alan Durrant.
In 1920, James Chadwick used a similar experimental setup to determine the Z value for a number of metals. The British physicist went on to discover the neutron in 1932, delineating it as a separate particle from the proton, the American Physical Society said .
What did the Rutherford model get right and wrong?
Yet the Rutherford model shared a critical problem with the earlier plum pudding model of the atom: The orbiting electrons in both models should be continuously emitting electromagnetic energy, which would cause them to lose energy and eventually spiral into the nucleus. In fact, the electrons in Rutherford's model should have lasted less than 10^-5 seconds.
Another problem presented by Rutherford's model is that it doesn't account for the sizes of atoms.
Despite these failings, the Rutherford model derived from the Geiger-Marsden experiments would become the inspiration for Niels Bohr 's atomic model of hydrogen , for which he won a Nobel Prize in Physics .
Bohr united Rutherford's atomic model with the quantum theories of Max Planck to determine that electrons in an atom can only take discrete energy values, thereby explaining why they remain stable around a nucleus unless emitting or absorbing a photon, or light particle.
Thus, the work of Rutherford, Geiger (who later became famous for his invention of a radiation detector) and Marsden helped to form the foundations of both quantum mechanics and particle physics.
Rutherford's idea of firing a beam at a target was adapted to particle accelerators during the 20th century. Perhaps the ultimate example of this type of experiment is the Large Hadron Collider near Geneva, which accelerates beams of particles to near light speed and slams them together.
- See a modern reconstruction of the Geiger-Marsden gold foil experiment conducted by BackstageScience and explained by particle physicist Bruce Kennedy .
- Find out more about the Bohr model of the atom which would eventually replace the Rutherford atomic model.
- Rutherford's protege Hans Gieger would eventually become famous for the invention of a radioactive detector, the Gieger counter. SciShow explains how they work .
Thomson's Atomic Model , Lumens Chemistry for Non-Majors,.
Rutherford Model, Britannica, https://www.britannica.com/science/Rutherford-model
Alpha particle, U.S NRC, https://www.nrc.gov/reading-rm/basic-ref/glossary/alpha-particle.html
Manners. J., et al, 'Quantum Physics: An Introduction,' Open University, 2008.
Durrant, A., et al, 'Quantum Physics of Matter,' Open University, 2008
Ernest Rutherford, Britannica , https://www.britannica.com/biography/Ernest-Rutherford
Niels Bohr, The Nobel Prize, https://www.nobelprize.org/prizes/physics/1922/bohr/facts/
House. J. E., 'Origins of Quantum Theory,' Fundamentals of Quantum Mechanics (Third Edition) , 2018
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Robert Lea is a science journalist in the U.K. who specializes in science, space, physics, astronomy, astrophysics, cosmology, quantum mechanics and technology. Rob's articles have been published in Physics World, New Scientist, Astronomy Magazine, All About Space and ZME Science. He also writes about science communication for Elsevier and the European Journal of Physics. Rob holds a bachelor of science degree in physics and astronomy from the U.K.’s Open University
The shape of light: Scientists reveal image of an individual photon for 1st time ever
Is light a particle or a wave?
New thunderstorms wider than Earth are spewing out green lightning on Jupiter — and could make one of the gas giant's massive bands disappear
Most Popular
- 2 Ancient 'land bridge' that connected Siberia to US wasn't what it seems, scientists find
- 3 Large, ghostly white crab-like predator discovered at the bottom of the Atacama Trench
- 4 New quantum computing milestone smashes entanglement world record
- 5 How long does it take to travel to the moon?
Rutherford model of the atom
Ernest Rutherford conducted an experiment where he fired alpha particles at a thin gold foil. He expected the particles to pass through the foil with only slight deflection based on the plum pudding atomic model. However, he discovered that some particles were deflected at large angles or reflected straight back, indicating the presence of a small, dense nucleus. This led Rutherford to propose the nuclear model of the atom with positive charge and mass concentrated at the center. Read less

More Related Content
- 1. Rutherford Rutherford Ernest Rutherford (1871-1937) PAPER PAPER • Learned physics in J.J. Thomson’ lab. • Noticed that ‘alpha’ particles were sometime deflected by something in the air. • Gold-foil experiment Animation by Raymond Chang – All rights reserved.
- 2. Rutherford ‘Scattering’ • In 1909 Rutherford undertook a series of experiments • He fired α (alpha) particles at a very thin sample of gold foil • According to the Thomson model the α particles would only be slightly deflected • Rutherford discovered that they were deflected through large angles and could even be reflected straight back to the source Lead collimator Gold foil α particle source θ
- 3. Rutherford’s Apparatus Rutherford received the 1908 Nobel Prize in Chemistry for his pioneering work in nuclear chemistry. beam of alpha particles radioactive substance circular ZnS - coated fluorescent screen gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
- 4. Rutherford’s Apparatus beam of alpha particles radioactive substance fluorescent screen circular - ZnS coated gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
- 5. Geiger-Muller Counter Hans Geiger Speaker gives “click” for each particle Window Particle path Argon atoms
- 6. Geiger Counter Ionization of fill gas takes place along track of radiation (-) Speaker gives “click” for each particle (+) Metal tube (negatively charged) + e- Window e- + + + e- e- Ionizing Free e- are attracted to radiation (+) electrode, completing path the circuit and generating a current. The Geiger Atoms or molecules Central wire electrode counter then translates of fill gas (positively charged) the current reading into a measure of radioactivity. Wilbraham, Staley, Matta, Waterman, Chemistry, 2002, page 857
- 7. What he expected…
- 8. What he got… richocheting alpha particles
- 9. The Predicted Result: expected path expected marks on screen Observed Result: mark on screen likely alpha particle path
- 10. Interpreting the Observed Deflections . . . . beam of . . undeflected . alpha . particles particles . . . . . . . deflected particle . . gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
- 11. Rutherford Scattering (cont.) Rutherford interpreted this result by suggesting that the α particles interacted with very small and heavy particles Case A Particle bounces off of atom? Case B Particle goes through atom? Case C Particle attracts to atom? Case D . Particle path is altered as it passes through atom?
- 12. Table: hypothetical description of alpha particles (based on properties of alpha radiation) observation hypothesis alpha rays don’t diffract ... alpha radiation is a stream of particles alpha rays deflect towards a negatively ... alpha particles have a positive charge charged plate and away from a positively charged plate alpha rays are deflected only slightly by ... alpha particles either have much an electric field; a cathode ray passing lower charge or much greater mass through the same field is deflected than electrons strongly Copyright © 1997-2005 by Fred Senese
- 13. Explanation of Alpha-Scattering Results Alpha particles Nucleus + + - - + + - + - + - + - - + - Plum-pudding atom Nuclear atom Thomson’s model Rutherford’s model
- 14. Results of foil experiment if plum- pudding had been correct. Electrons scattered throughout positive charges + - + - + + - + - - + + + - - - Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 57
- 15. Interpreting the Observed Deflections deflected particle . . . . beam of . . undeflected . alpha . particles particles . . . . . . . . . gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
- 16. Rutherford’s Gold-Leaf Experiment Conclusions: Atom is mostly empty space Nucleus has (+) charge Electrons float around nucleus Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
- 17. • Hit moth driving car – no change in car direction • Hit deer – car changes direction Alpha particle moth Gold Atom deer Large angle of deflection, must have hit massive object!
Editor's Notes
- Objectives: To describe the Rutherford nuclear model of the atom. To state the relative charge and approximate mass of the electron, proton, and neutron.
- Ernest Rutherford received the Nobel Prize in chemistry (1908) for his work with radioactivity. Ernest Rutherford (1871-1937) was born in Nelson, New Zealand in 1871. He began work in J.J. Thompson ’s laboratory in 1895. He later moved to McGill University in Montreal where he became one of the leading figures in the field of radioactivity. From 1907 on he was professor at the University of Manchester where he worked with Geiger and Marsden. He was awarded the Nobel Prize for Chemistry in 1908 for his work on radioactivity. In 1910, with co-workers Geiger and Marsden he discovered that alpha-particles could be deflected by thin metal foil. This work enabled him to propose a structure for the atom. Later on he proposed the existence of the proton and predicted the existence of the neutron. He died in 1937 and like J.J. Thompson is buried in Westminster Abbey. He was one of the most distinguished scientists of his century. Is the Nucleus Fundamental? Because it appeared small, solid, and dense, scientists originally thought that the nucleus was fundamental. Later, they discovered that it was made of protons (p+), which are positively charged, and neutrons (n), which have no charge.
- Rutherford’s results strongly suggested that both the mass and positive charge are concentrated in a tiny fraction of the volume of the atom, called the nucleus. Rutherford established that the nucleus of the hydrogen atom was a positively charged particle, which he called a proton. Also suggested that the nuclei of elements other than hydrogen must contain electrically neutral particles with the same mass as the proton. The neutron was discovered in 1932 by Rutherford’s student Chadwick. Because of Rutherford’s work, it became clear that an α particle contains two protons and neutrons—the nucleus of a helium atom.
- MODERN ALCHEMY “ Ernest Rutherford (1871-1937) was the first person to bombard atoms artificially to produce transmutated elements. The physicist from New Zealand described atoms as having a central nucleus with electrons revolving around it. He showed that radium atoms emitted “rays” and were transformed into radon atoms. Nuclear reactions like this can be regarded as transmutations – one element changing into another, the process alchemists sought in vain to achieve by chemical means.” Eyewitness Science “Chemistry” , Dr. Ann Newmark, DK Publishing, Inc., 1993, pg 35 When Rutherford shot alpha particles at a thin piece of gold foil, he found that while most of them traveled straight through, some of them were deflected by huge angles.
- MODERN ALCHEMY Ernest Rutherford (1871-1937) was the first person to bombard atoms artificially to produce transmutated elements. The physicist from New Zealand described atoms as having a central nucleus with electrons revolving around it. He showed that radium atoms emitted “rays” and were transformed into radon atoms. Nuclear reactions like this can be regarded as transmutations – one element changing into another, the process alchemists sought in vain to achieve by chemical means. Eyewitness Science “Chemistry” , Dr. Ann Newmark, DK Publishing, Inc., 1993, pg 35 Ernest Rutherford English physicist. (1910) Wanted to see how big atoms are. Used radioactivity, alpha particles - positively charged pieces given off by polonium atoms. Shot them at a thin gold foil (~0.5 um thick) which can be made a few atoms thick. When the alpha particles hit a florescent screen, it glows. Approximately 1/20,000 bounced back at the alpha emitter source. Rutherford said this was like shooting a 15" shell at tissue paper and the shell came back and hit you. It was clearly, NOT what he thought should happen if Thomson's model of the atom was correct. Ernest Rutherford received the 1908 Nobel prize in chemistry for his work at McGill University with radioactive substances.
- Radiation cannot be seen, heard, felt, or smelled. Thus warning signs and radiation detection instruments must be used to alert people to the presence of radiation and to monitor its level. The Geiger counter is one such instrument that is widely used. Other devices used to detect and measure ionizing radiation : scintillation counter, film badge Free e- are attracted to (+) electrode, completing the circuit and generating a current. The Geiger counter then translates the current reading into a measure of radioactivity.
- Ernest Rutherford English physicist. (1910) Wanted to see how big atoms are. Used radioactivity, alpha particles - positively charged pieces given off by polonium. Shot them at gold foil which can be made a few atoms thick. When the alpha particles hit a florescent screen, it glows.
- The observations : (1) Most of the alpha particles pass through the foil un-deflected. (2) Some alpha particles are deflected slightly as the penetrate the foil. (3) A few (about 1 in 20,000) are greatly deflected. (4) A similar small number do not penetrate the foil at all, but are reflected back toward the source. Rutherford believed that when positively charged alpha particles passed near the positively charged nucleus, the resulting strong repulsion caused them to be deflected at extreme angles. Rutherford's interpretation : If atoms of the foil have a massive, positively charged nucleus and light electrons outside the nucleus, one can explain how: (1) an alpha particle passes through the atom un-deflected (a fate share by most of the alpha particles); (2) an alpha particle is deflected slightly as it passes near an electron; (3) an alpha particle is strongly deflected by passing close to the atomic nucleus; and (4) an alpha particle bounces back as it approaches the nucleus head-on.
- In the first case, one would assume the alpha particle (positively charged) struck another positively charged particle. Perhaps William Thomson (Lord Kelvin) was correct and the atom is like plum-pudding and is a positive ball with electrons embedded. In the middle example, where the alpha particles pass straight through and are not deflected, it implies the atom is mostly empty space or the alpha particle is too penetrating to give any useful information about the composition of an atom. The third example is NOT what is observed. For this to occur, the atom would have to be negatively charged and absorb all the positively charged alpha particles. At some point the atom would be “full” of alpha particles and then the atom would begin to bounce off of its surface alpha particles. The last example also occurs. In the gold foil experiment, Rutherford observed case A and D (rarely) and mostly case B. This was explained by saying the atom was mostly empty space where electrons spin rapidly around a positively charged, massive (most of the mass of the atom) but tiny nucleus.
- Atom is mostly empty Small dense, positive piece at center (the nucleus). Alpha particles are deflected by it… if they get close enough to nucleus. Conclusion: From Rutherford’s results he proposed a nuclear atom model where there is a dense center of positive charge called the nucleus around which electrons move in space that is otherwise empty.
- “ Rutherford’s Gold-Leaf Experiment” Description This slide illustrates Ernest Rutherford’s experiment with alpha particles and gold foil and his interpretation of the results. Basic Concepts When charged particles are directed at high speed toward a metal foil target, most pass through with little or no deflection, but some particles are deflected at large angles. Solids are composed of atoms that are closely packed. The atoms themselves are mostly empty space. All atoms contain a relatively small, massive, positively charged nucleus. The nucleus is surrounded by negatively charged electrons of low mass that occupy a relatively large volume. Teaching Suggestions Use this slide to describe and explain Rutherford’s experiment. Rutherford designed the apparatus shown in figure (A) to study the scattering of alpha particles by gold. Students may have difficult with the concepts in this experiment because they lack the necessary physics background. To help students understand how it was determined that the nucleus is relatively massive, use questions 3 and 4 to explain the concept of inertia. Explain that the electrostatic force is directly proportional to the quantity of electric charge involved. A greater charge exerts a greater force. (Try comparing the electrostatic force to the foce of gravity, which is greater near a massive object like the sun, but smaller near an object of lesser mass, such as the moon.) The force exerted on an alpha particle by a concentrated nucleus would be much greater that the force exerted on an alpha particle by a single proton. Hence, larger deflections will result from a dense nucleus than from an atom with diffuse positive charges. Point out that Rutherford used physics to calculate how small the nucleus would have to be produce the large-angle deflections observed. He calculated that the maximum possible size of the nucleus is about 1/10,000 the diameter of the atom. Rutherford concluded that the atom is mostly space. Questions If gold atoms were solid spheres stacked together with no space between them, what would you expect would happen to particles shot at them? Explain your reasoning. When Ernest Rutherford performed the experiment shown in diagram (A) he observed that most of the alpha particles passed straight through the gold foil. He also noted that the gold foil did not appear to be affected. How can these two observations be explained? Can you explain why Rutherford concluded that the mass of the f\\gold nucleus must be much greater than the mass of an alpha particle? (Hint: Imagine one marble striking another marble at high speed. Compare this with a marble striking a bowling ball.) Do you think that, in Rutherford’s experiment, the electrons in the gold atoms would deflect the alpha particles significantly? Why or why not? (Hint: The mass of an electron is extremely small.) Rutherford experimented with many kinds of metal foil as the target. The results were always similar. Why was it important to do this? A friend tries to convince you that gold atoms are solid because gold feels solid. Your friend also argues that, because the negatively charged electrons are attracted to the positively charged nucleus, the electrons should collapse into the nucleus. How would you respond? As you know, like charges repel each other. Yet, Rutherford determined that the nucleus contains all of an atom’s positive charges. Invent a theory to explain how all the positive charges can be contained in such a small area without repelling each other. Be creative!
- http://particleadventure.org/frameless/modern_atom.html

IMAGES
VIDEO