- Edit account
- United States
- Czech Republic
- Netherlands
- New Zealand
- Philippines
- Switzerland
- United Kingdom

Global Point of Care


VALUE ASSIGNMENT & ELECTRONIC VALUE ASSIGNMENT SHEETS (VAS/ eVAS )

SELECT A PRODUCT, VAS/ eVAS TYPE, AND SOFTWARE VERSION FROM THE DROP-DOWN MENUS.
- SELECT DEVICE
- i-STAT ALINITY
- SELECT VAS TYPE
VALUE ASSIGNMENT SHEETS
- ELECTRONIC VALUE ASSIGNMENT SHEETS
- SELECT VAS CLEW
- SELECT eVAS CLEW
Use Value Assignment Sheets (VAS) to locate the correct target values and ranges for your i-STAT System test cartridge controls and calibration verification materials. You may also access electronic Value Assignment Sheets (eVAS).
i-STAT 1 content updated 11 December 2024
To find the correct VAS and ranges, you need to:
- Locate the cartridge type and lot number found on your cartridge box or pouch.
- Choose the corresponding lot and type from the menu below.
- Identify the corresponding cartridge type and lot prefix letter WITHIN the value assignment sheet.
CLEW A49 EXPIRES 18 JUNE 2025
Act level 1 control.
- Lot 261168, for CLEW A49
- Lot 261171, for CLEW A49
- Lot 261173, for CLEW A49
- Lot 261175, for CLEW A49
ACT LEVEL 2 CONTROL
- Lot 271169, for CLEW A49
- Lot 271171, for CLEW A49
- Lot 271173, for CLEW A49
- Lot 271175, for CLEW A49
β-Hcg Calibration Verification Set
- Lot 230039, for CLEW A49
- Lot 230104, for CLEW A49
- Lot 240129, for CLEW A49
- Lot 240332, for CLEW A49
β-HCG LEVEL 1 CONTROL
- Lot 351169, for CLEW A49
- Lot 351171, for CLEW A49
- Lot 351178, for CLEW A49
- Lot 351184, for CLEW A49
β-HCG LEVEL 2 CONTROL
- Lot 361169, for CLEW A49
- Lot 361171, for CLEW A49
- Lot 361178, for CLEW A49
- Lot 361184, for CLEW A49
β-HCG LEVEL 3 CONTROL
- Lot 371169, for CLEW A49
- Lot 371171, for CLEW A49
- Lot 371178, for CLEW A49
- Lot 371184, for CLEW A49
BNP Calibration Verification Set
- Lot 230258, for CLEW A49
- Lot 230354, for CLEW A49
- Lot 240060, for CLEW A49
- Lot 240171, for CLEW A49
BNP LEVEL 1 CONTROL
- Lot 041176, for CLEW A49
- Lot 041179, for CLEW A49
- Lot 041182, for CLEW A49
- Lot 041185, for CLEW A49
BNP LEVEL 2 CONTROL
- Lot 051176, for CLEW A49
- Lot 051179, for CLEW A49
- Lot 051182, for CLEW A49
- Lot 051185, for CLEW A49
BNP LEVEL 3 CONTROL
- Lot 061176, for CLEW A49
- Lot 061179, for CLEW A49
- Lot 061182, for CLEW A49
- Lot 061185, for CLEW A49
CHEM8+ CALIBRATION VERIFICATION LEVEL 1B
- Lot 181169, for CLEW A49
- Lot 181174, for CLEW A49
CK-MB Calibration Verification Set
- Lot 230055, for CLEW A49
- Lot 230132, for CLEW A49
- Lot 230282, for CLEW A49
- Lot 240155, for CLEW A49
CK-MB LEVEL 1 CONTROL
- Lot 071169, for CLEW A49
- Lot 071172, for CLEW A49
- Lot 071177, for CLEW A49
- Lot 071185, for CLEW A49
CK-MB LEVEL 2 CONTROL
- Lot 081169, for CLEW A49
- Lot 081172, for CLEW A49
- Lot 081177, for CLEW A49
- Lot 081185, for CLEW A49
CK-MB LEVEL 3 CONTROL
- Lot 091169, for CLEW A49
- Lot 091172, for CLEW A49
- Lot 091177, for CLEW A49
- Lot 091185, for CLEW A49
Ctni Calibration Verification Set
- Lot 230100, for CLEW A49
- Lot 230244, for CLEW A49
- Lot 240357, for CLEW A49
- Lot 240068, for CLEW A49
- Lot 240079, for CLEW A49
- Lot 240220, for CLEW A49
CTNI LEVEL 1 CONTROL
- Lot 011171, for CLEW A49
- Lot 011176, for CLEW A49
- Lot 011179, for CLEW A49
- Lot 011182, for CLEW A49
- Lot 011187, for CLEW A49
CTNI LEVEL 2 CONTROL
- Lot 021171, for CLEW A49
- Lot 021176, for CLEW A49
- Lot 021180, for CLEW A49
- Lot 021182, for CLEW A49
- Lot 021187, for CLEW A49
CTNI LEVEL 3 CONTROL
- Lot 031171, for CLEW A49
- Lot 031176, for CLEW A49
- Lot 031179, for CLEW A49
- Lot 031182, for CLEW A49
- Lot 031187, for CLEW A49
i-STAT CALIBRATION VERIFICATION SET
- Lot 23307, for CLEW A49
- Lot 24050, for CLEW A49
- Lot 24211, for CLEW A49
i-STAT LEVEL 1 CONTROL
- Lot 101170, for CLEW A49
- Lot 101179, for CLEW A49
i-STAT LEVEL 2 CONTROL
- Lot 111170, for CLEW A49
- Lot 111174, for CLEW A49
- Lot 111179, for CLEW A49
i-STAT LEVEL 3 CONTROL
- Lot 121170, for CLEW A49
- Lot 121174, for CLEW A49
- Lot 121179, for CLEW A49
PT plus LEVEL 1 CONTROL
- Lot 501170, for CLEW A49
- Lot 501174, for CLEW A49
PT plus LEVEL 2 CONTROL
- Lot 511170, for CLEW A49
- Lot 511174, for CLEW A49
PT LEVEL 1 CONTROL
- Lot 281168, for CLEW A49
- Lot 281172, for CLEW A49
PT LEVEL 2 CONTROL
- Lot 291168, for CLEW A49
- Lot 291172, for CLEW A49
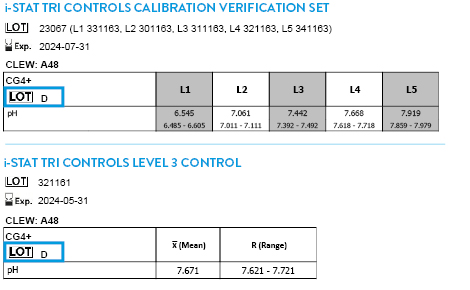
Tricontrols Calibration Verification Set
- Lot 23226, for CLEW A49
- Lot 23335, for CLEW A49
- Lot 24057, for CLEW A49
- Lot 24124, for CLEW A49
- Lot 24212, for CLEW A49
TRICONTROLS LEVEL 1 CONTROL
- Lot 301168, for CLEW A49
- Lot 301171, for CLEW A49
- Lot 301174, for CLEW A49
- Lot 301177, for CLEW A49
- Lot 301179, for CLEW A49
TRICONTROLS LEVEL 2 CONTROL
- Lot 311168, for CLEW A49
- Lot 311171, for CLEW A49
- Lot 311174, for CLEW A49
- Lot 311177, for CLEW A49
- Lot 311179, for CLEW A49
TRICONTROLS LEVEL 3 CONTROL
- Lot 321168, for CLEW A49
- Lot 321171, for CLEW A49
- Lot 321174, for CLEW A49
- Lot 321177, for CLEW A49
- Lot 321179, for CLEW A49
Additional Resources
Documentation


Instructions For Use
Electronic value assignment sheets.
You can automatically update your device(s) with the latest Value Assignment Sheet data to streamline and simplify the liquid quality control process.
i-STAT 1 DOWNLOAD INSTRUCTIONS
- Select the file for download and Save. Do not rename the file .
- Confirm “Save as type” is either:
- .VAS Document
FOR DE CUSTOMERS:
- Save the file to any directory accessible to the i-STAT/DE and click “Save”.
- Close the “Download Complete” window when finished.
- Access the DE Customization workspace:
Upload the eVAS file to i-STAT/DE:
- Click Update i-STAT/DE at the top of the Customization Workspace and select Upload Update File.
- Click Browse when the “Specify file for i-STAT/DE update” box opens.
- Navigate to the directory location where the eVAS file was saved.
- Select the eVAS file and click Open.
- Click “Upload.” A confirmation message will appear if the upload is successful.
eVAS File For Download
- APOC20243391.VAS
- SELECT eVAS CLEW
Use Value Assignment Sheets (VAS) to locate the correct target values and ranges for your i-STAT System test cartridge controls and calibration verification materials. You may also access electronic Value Assignment Sheets (eVAS).
i-STAT Alinity content updated 11 December 2024

CLEW D48 EXPIRES 15 JANUARY 2025
Tbi calibration verification set.
- Lot 240124, for CLEW D48
- Lot 240163, for CLEW D48
TBI Level 1 Control
- Lot 641172, for CLEW D48
- Lot 641173, for CLEW D48
TBI Level 2 Control
- Lot 651172, for CLEW D48
- Lot 651173, for CLEW D48
You can automatically update your device(s) with the latest Value Assignment Sheet data to streamline and simplify the liquid quality control process.
i-STAT ALINITY DOWNLOAD INSTRUCTIONS
You must customize your instrument before using eVAS. To do so, use AlinIQ CWi to create a profile with eVAS selected and enabled for use, then load the profile onto the instrument. For further instructions, see the Manage and Assemble Profiles section of the System Operations Manual.
To download eVAS package file:
- Delete all pre-existing eVAS package files from your computer’s Downloads directory.
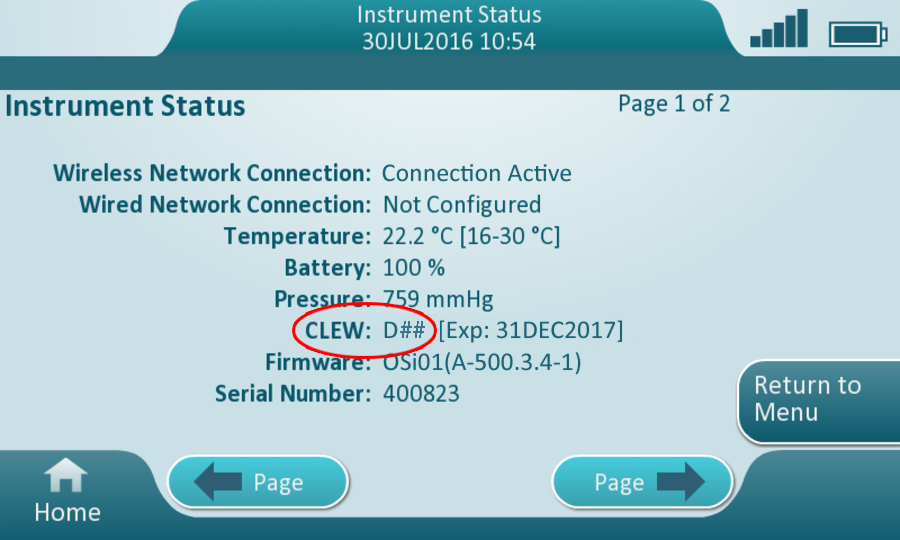
- Check the instrument CLEW.
- Power up the instrument.
- Touch More Options -> Instrument Status
- Observe CLEW indicated on the screen.
- Find the eVAS package file below that corresponds with the CLEW indicated on the Instrument Status screen.
- Download the eVAS package file.
- Click the eVAS Package File link corresponding to the instrument CLEW indicated on the screen. DO NOT RENAME THE FILE.
- Copy the eVAS package file to a USB memory stick.
- USB memory stick must be formatted as “FAT32” via Windows OS prior to use.
To install eVAS package file:
- From the Home screen: Touch More Options -> Quality Options -> Update eVAS -> Install from USB Follow prompts
i-STAT Alinity content updated 11 December 2024
- APOC20243393.apkg
CLEW D49 EXPIRES 16 JULY 2025
- Lot 240124, for CLEW D49
- Lot 240163, for CLEW D49
- Lot 641172, for CLEW D49
- Lot 641173, for CLEW D49
- Lot 651172, for CLEW D49
- Lot 651173, for CLEW D49
- APOC20243395.apkg
Stay informed
Sign up to receive valuable updates from Abbott.
A Leader In Rapid Point-of-care Diagnostics.
©2024 Abbott. All rights reserved. Unless otherwise specified, all product and service names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company.
This website is governed by applicable U.S. laws and governmental regulations. The products and information contained herewith may not be accessible in all countries, and Abbott takes no responsibility for such information which may not comply with local country legal process, regulation, registration and usage.
Your use of this website and the information contained herein is subject to our Website Terms and Conditions and Privacy Policy . Photos displayed are for illustrative purposes only. Any person depicted in such photographs is a model. GDPR Statement
Not all products are available in all regions. Check with your local representative for availability in specific markets. For in vitro diagnostic use only. For i-STAT test cartridge information and intended use, refer to individual product pages or the cartridge information (CTI/IFU) in the i-STAT Support area.
Abbott - A Leader in Rapid Point-of-Care Diagnostics.
Technical Support
For Technical Support telephone contact details and opening hours, please select a country from the dropdown.
Website Terms and Conditions and Privacy Policy: US Citizens | Non-US Citizens .

Value Assignment Program
It is recommended to use Third-Party control materials to ensure that the methods are performing properly. Quality control materials can be categorized as assayed or unassayed. Assayed controls are provided with a list of targeted values and upper and lower limits (control ranges) for all analytes specified in the control material for the common analytical methods and instruments. Unassayed controls have no assigned analyte values provided by the manufacturer. The control values for these materials must be determined by the individual laboratory.
More Diagnostics works closely with the instrument manufacturers and selected clinical laboratory partners to establish statistically meaningful data as part of the value-assignment process for each given lot number of quality control. The results are contained in the Value Assignment documents also accessible on our website.
Why Is Value Assignment Important?
What are the benefits?
Join our Value Assignment Team and make an impact in the laboratory to help ensure accuracy.
Contact Our Value Assignment Coordinator
Sign up for our newsletter to stay connected with our latest news and updates
Quality Control Value Assignment Program
Contribute to a diverse global testing network
Bio-Rad is a leader in independent quality controls with the world’s largest QC peer group program. QC Value Assignment testing is critical in contributing meaningful data to support our diverse global QC customers and plays an integral part in the release of new assayed quality control lots. When participating in the QC Value Assignment Program, clinical laboratories and other organizations can earn money while contributing data toward establishing analyte recovery means and ranges for Bio-Rad quality control inserts. Together with our industry-leading Unity data management software, our QC Value Assignment Program can help laboratories increase confidence in the reliability of patient test results.
Inclusive QC value assignment program for testing
Enhanced support for worldwide peer group.
Value assignment testing is critical to support our diverse global testing network. By participating in the VA program, your laboratory or organization can help play a key role in new product development or product modification studies.
Help generate product insert values
By participating in the value assignment program, labs contribute data that is used to develop product insert values. Submitting data in the VA program helps increase visibility of popular instruments in your region.
Added revenue for your lab
Participation in the QC value assignment program can also generate additional revenue for your laboratory or organization. We seek and engage support from high-quality labs, and labs are compensated for these testing services.
Getting started
In order to join the QC value assignment program, there are some minimum requirements for participation, including:
- Your lab must be certified. (In the US, ISO, CLIA/CAP, or Joint Commission is required. In other countries, local or regional certification may be acceptable.)
- Results must be delivered within two weeks from receipt of sample.
- Your lab must be able to directly enter results into Bio-Rad's online database, VAWeb.
- Each participating lab shall maintain its current profile and contact information in VAWeb.
How it works
Bio-Rad assesses the lab for fit in the program.
If accepted, lab enrolls in the program and receives samples.
Lab confirms receipt of samples in VAWeb.
Lab performs testing and enters data into VAWeb.
Benefits of the QC value assignment program
Data is used to develop product insert values
Diverse VA network helps in contributing meaningful data for each control lot
Joining the program increases your lab’s revenue
Unity software offers world’s largest peer network
Bio-Rad quality controls
Bio-Rad offers more than 300 independent quality control products with over 600 of these analytes tested in most areas of the laboratory. Our controls are used to monitor precision on hundreds of the most widely used diagnostic test systems.
InteliQ Load-and-Go
Cardiac Markers
Coagulation
Diabetes & Hemoglobin
Immunoassay
Infectious Disease
Therapeutic Drug Monitoring
Serum Indices
Experience the Bio-Rad advantage
Integrated unity software solutions.
By partnering with Bio-Rad, your lab can access the powerful Unity QC data management system, which provides access to the world’s largest peer group through our interlaboratory program. Assessment tools and reports can help your lab meet compliance requirements, streamline your QC workflow, create criteria for testing and benchmarking lab performance, and report results with confidence.
Highest level of customer service, partnership, and technical support
Bio-Rad can support your lab with more than our leading independent quality controls. We provide QC technical support, program support for both Unity and the QCNet portal, software and connectivity support, and specialized technical proficiency in the form of trained specialists.
Frequently asked questions
What is the qc value assignment program.
Participation in the QC Value Assignment Program with other clinical laboratories and hospitals allows you to contribute meaningful data towards establishing analyte statistical means and ranges posted for Bio-Rad quality controls.
What is the benefit of participating in QC value assignment to your lab or organization?
- Generate additional revenue for your organization
- Increase visibility for instruments and assays utilized by your laboratory
- Contribute with your meaningful data to support a diverse global testing network
How can your lab participate?
Your privacy matters.
Before you visit, we want to let you know we use cookies to offer you a better browsing experience. To learn more about how we use cookies, please review our Cookie Policy, accessible from the Manage Preferences link below. We would appreciate your confirmation by either accepting all cookies or by declining and managing your cookie preferences under the Manage Preferences link below.
Cookie Preferences
We use various types of cookies to enhance and personalize your browsing experience on our website. You may review the various types in the descriptions below and decide which cookie preferences you wish to enable. If you wish to decline all non-essential cookies, you may browse our site using strictly-necessary cookies. To learn more about how we use cookies, please visit our Cookie Policy .
Strictly-Necessary Cookies
These cookies are essential for our website to function properly. They either serve as the sole purpose of carrying out network transmissions or they allow you to browse and use features, such as accessing secure areas of the site. These cookies are strictly necessary because services like the shopping cart and invoicing cannot be provided without these cookies. Since these cookies are strictly necessary in order for our website to function, no consent is required to enable them. If you wish to disable these cookies, please update your settings under your browser’s preferences. If these cookies are disabled, please be aware that you will not be able to access certain features of the site like purchasing online.
Functionality Cookies
These cookies improve your browsing experience and provide useful, personalized features. They are used to remember selections that you have made such as your preferred language, region, and username. They also remember changes that you made in text sizes, fonts, and other customizable parts of the Web. Together, this information allows us to personalize features on our website in order to provide you with the best possible browsing experience. The information that these cookies collect is anonymous and cannot track your activity on other websites.
Analytics Cookies
These cookies are used to help ensure that your browsing experience is optimal. They collect anonymous data on how you use our website in order to build better, more useful pages. For instance, we can recognize and count the number of visitors, see how visitors moved around the site, and we can identify which pages returned error messages. This information enables us to enhance your experience and helps us troubleshoot any issues that prevented you from reaching the content that you needed. In order to improve the performance of our site, we use products such as WebTrends OnDemand and Google Analytics to track site usage. You can find the list of products that we use to collect information that is relevant to Analytics Cookies here:
- Google Analytics
- Adobe Analytics
- WebTrends On Demand
Targeting or Advertising Cookies
These cookies are used to deliver personalized content based on your interests through third-party ad services. This allows us to improve your online experience by helping you find products that are relevant to your interests faster. They remember websites that you have visited and the information is shared with other organizations such as advertisers. These cookies are also used to limit the number of times you see an ad and help measure the effectiveness of a marketing campaign. You can find the list of products that we use to collect information that is relevant to Advertising Cookies here:
- Doubleclick
QCNet International
Existing QCNet international sites may be accessed through the following links:
- Canada (English)
- Canada (Français)
- Czech Republic
- Latin America
- United Kingdom

Also available from your favorite distributor CLIAwaived • Cardinal Health • Henry Schein • Medline McKesson • Whitmire Medical • Fisher Scientific Universal Marine Med Supply • STAT Technologies Cenmed (Military–DOD–GOV) • Therapak (Avantor)


An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
https://www.nist.gov/publications/definitions-terms-and-modes-used-nist-value-assignment-reference-materials-chemical-1
Definitions of Terms and Modes Used at NIST for Value-Assignment of Reference Materials for Chemical Measurements
Additional citation formats.
- Google Scholar
If you have any questions about this publication or are having problems accessing it, please contact [email protected] .

- Build Survey
- Question types
- Value assignment (professional user)
Value assignment: How can I use it?

The Value Assignment element is a very powerful tool for complex surveys. This also means it’s quite complicated to explain! To do this, we’ve created four examples of how to use it.

For the element with the first rank in a rank order question, no placeholder exists. To get a placeholder for a later question we have to create it. To do this:
- Create a text variable (in this example we’ll call it ‘rank_one’)
- The setting should be ‘Execute everytime the participant passes this point’
- The value of ‘rank_one’ should be set to the label of the corresponding element
- The filter should be set to: Execute only if the corresponding element has rank one

Now you can use the placeholder {{custom.rank_one}} for a question such as ‘Why did you choose {{custom.rank_one}} as rank one?’

Use Case 2: Random Number
Let’s imagine you want to show one of four questions, but the question should be random. In this case it would be useful to have a random number created and stored.
To do this:

- Create a number variable (in this example we’ll call it ‘random_number’)
- The setting should be ‘Execute only the first time the participant passes this point’
- The value of ‘random_number’ should be set to randomInt(1,5). This function will create a random number between 1 and 4 (the maximum value is excluded).

Now you can use this ‘random_number’ variable as the filter variable for the four questions. You should set a filter like: ‘Show this element if ‘random_number’ is equal to 1′.
Use Case 3: Test Calculation

Let’s imagine you want to use Survalyzer as a tool to conduct a test, for example, for a school class. On the final page you want to show the number of points scored, with a corresponding grade. For this to work, we need some preparation. For each question we will need to create an own number variable. We will also need to create two value assignments per question (only if the participant is allowed to go backwards):

- one value assignment to set the variable to 0 (which happens when the participant passes this element)
- a filtered value assignment which sets the value to the number of points the participant gets for this question; filtered to only assign this value if the question is answered correctly. The setting should be ‘everytime the participant passes this element’.
At the end of the survey we’ll create an additional variable, let’s call it ‘sum_points’, where we will store the sum of all question variables. For example: pq1+pq2+pq3…etc.

- a filtered value assignment which sets the value to the sum of all points; the setting should be ‘every time the participant passes this point’.
Now we have a sum of all points attained, stored in a variable. For each grade we now need to create a text block. Here we’ll use our ‘sum_points’ variable as a filter variable and placeholder, to show the number of points attained. Now we have a working test in place.
Use Case 4: Saving complex filters
Sometimes you may need to set a complex filter several times at different places in your survey. There is a way to make this easier.
To do this you’ll need to create two value assignments (only if the participant is allowed to go backwards) directly after you gathered all necessary information for your complex filter:

- one value assignment to set the variable (let’s call it ‘complex_filter’) to 0 (which happens whenever the participant passes this element)
- The Filter to set is your complex filter

Now you can simply use the filter ‘show this element if ‘complex_filter’ is equal to 1′. This is much easier to set than repeatedly creating a complex filter. Additionally, if you need to update the filter, you only have to change it in one place.
Related Articles
- Enable and disable text fields when selecting answer in previous question
- addDays: adding days to today’s date
- Setting quotas on answer options
- Set maximum time to answer survey
- GetSurveyLink(ID): Generate personal link
- Count: counting characters of a text
- Skip to main content

QC VALUE ASSIGNMENT PROGRAM
Your laboratory will receive monetary compensation by signing-up to be a participant in our Value Assignment Program. Quantimetrix value assignment is a vital requirement when releasing new lots of quality control material or introducing new or enhanced products.
- Extra cash for your laboratory
- Data results are evaluated to establish ranges for product inserts
- Sign-up and become a front line evaluator for new or enhanced products
Please complete the form and click "submit." Participants must be CLIA certified or certified with an equivalent agency.

QC VALUE ASSIGNMENT PROGRAM FORM
Please click here to access the form. Once you've completed it, click “submit”.
Participants must be CLIA certified or certified with an equivalent agency.
- Your cart is empty! Return to shop
Forgotten Password
Quantrol Users Login Here

IMAGES
COMMENTS
Dec 11, 2024 · Use Value Assignment Sheets (VAS) to locate the correct target values and ranges for your i-STAT System test cartridge controls and calibration verification materials. You may also access electronic Value Assignment Sheets (eVAS).
Join our Value Assignment Team and make an impact in the laboratory to help ensure accuracy. Providing Premier Clinical Quality Controls. Meeting the needs of clinical laboratories and manufacturing partners for over 30 years.
The Value Assignment Web provides a secure and automated system to submit value assignment data over the Internet. Ensures all data is evaluated against uniform standards and increases the efficiency of the analysis process by highlighting suspicious data.
QC value assignment testing supports a diverse global network of clinical labs; help establish statistically valid means and ranges for Bio-Rad quality control inserts.
Control Value Assignment Ranges and Verification Mean Values; Analyzer Validation for Your CLIA Inspection; Order Form; Custom Product Development; Terms & Conditions; Contact Us; Choose a lot number: Lot #528023003 (Rev. 09/18/2023) Lot #465023002 (Rev. 09/18/2023) Lot #465023001 (Rev. 09/06/2023)
Jan 1, 2000 · certification, chemical measurements, modes, standard reference materials, value-assignment
Value assignment is an important requirement when rolling over to a new lot of QC material. The ability to provide full coverage of instrument target values involves the partnership of Thermo Fisher Scientific with laboratories performing the required testing.
Liquichek Immunoassay Plus Control is a liquid, human serum-based control that covers a vast array of today's most popular routine immunoassay analytes. Assigned values are available for all major automated analyzers, making it a highly efficient solution for laboratories that focus on routine tests.
Nov 15, 2022 · The Value Assignment element is a very powerful tool for complex surveys. This also means it’s quite complicated to explain! To do this, we’ve created four examples of how to use it.
Your laboratory will receive monetary compensation by signing-up to be a participant in our Value Assignment Program. Quantimetrix value assignment is a vital requirement when releasing new lots of quality control material or introducing new or enhanced products.